Simple blood test could reveal likelihood of deadly skin cancer returning, study suggests
A new study finds that fragments of tumor DNA in a patient's bloodstream could show that they are at high risk of a melanoma recurrence.
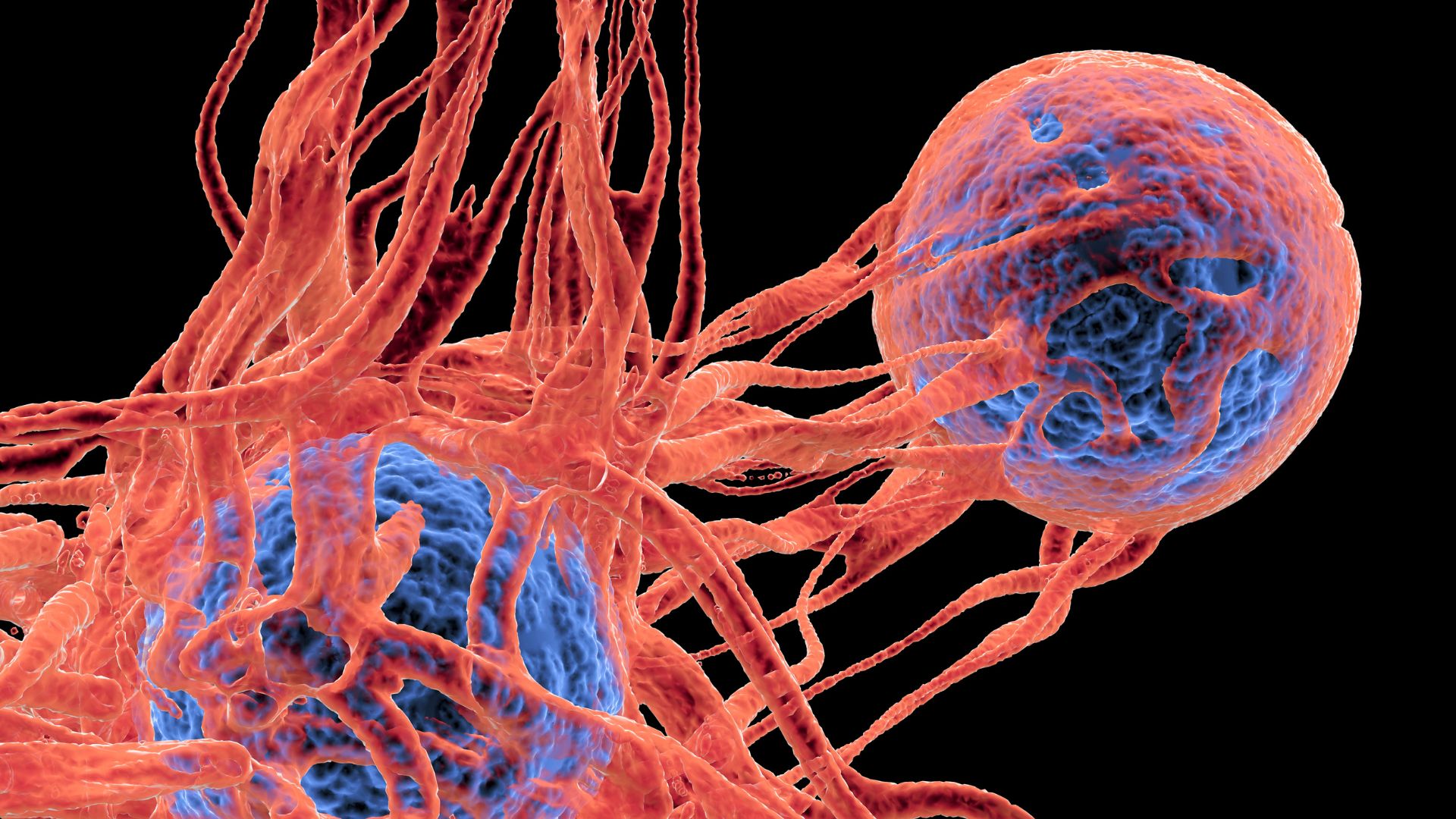
A simple blood test could reveal who is at high risk of skin cancer recurrence after tumor-removal surgery.
The test can detect fragments of tumor DNA with a simple blood draw to reveal the lingering presence of Stage III melanoma — a metastatic form of the deadliest form of skin cancer — that can't be seen with CT scans. Although the test isn't perfect, it could help flag patients who need aggressive treatment because their cancer is likely to come back.
"We're envisioning the test being used to monitor patients over time (perhaps every month or couple of months in the first 1-3 years after surgery) for an early indication that the melanoma is recurring," study senior author Dr. David Polsky, a dermatologic oncologist at the New York University Grossman School of Medicine, told Live Science in an email.
If the test showed signs of tumor DNA, Polsky continued, the doctor might choose to use more advanced imaging techniques to search for small, easy-to-miss tumors, or they might move to a more aggressive treatment regimen that uses a combination of cancer drugs instead of just one, for example.
Related: Simple blood tests could be the future of cancer diagnosis
Melanoma is a cancer of melanocytes, a type of pigmented skin cell. It accounts for only 1% of skin cancers, but it causes the most skin cancer deaths because it can quickly spread to other organs, or metastasize. Early detection is one of the best ways to boost the likelihood of survival.
Polsky and his colleagues focused on Stage III melanoma, which is melanoma that has spread to nearby lymph nodes, where immune cells are made and stored. Doctors perform surgery to remove as much of the cancer as possible before starting medications to kill any remaining tumor cells.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Patients then get CT scans to look for any signs of recurrence, but some patients have tiny deposits of melanoma that are too small to be detected by CT. To catch those deposits earlier, Polsky and his team turned to circulating tumor DNA, or ctDNA. These are DNA fragments released from tumor cells during their normal life cycle. The fragments circulate in the plasma — the liquid portion of the blood — and can be detected by telltale mutations that are unique to cancer.
As part of a larger clinical trial of a combination of cancer drugs, the research team studied blood samples from 597 patients who had recently undergone surgery. The participants also had follow-up blood samples taken three months, six months, nine months and 12 months after either starting a treatment or receiving a placebo.
Immediately after surgery, 13% of the patients had detectable ctDNA in their blood plasma. Every single one of these patients experienced a cancer recurrence, the researchers found. Patients were also more likely to see their melanoma return when their ctDNA rose during the follow-up tests or if the ctDNA remained persistently high over the course of the testing.
The presence of the ctDNA predicted the return of the cancer 100% of the time; no one with a positive test escaped melanoma relapse. But the absence of ctDNA did not mean the patients were out of the woods. A negative test was correct 71% of the time in predicting that the person's cancer would not return. But some patients with no detectable ctDNA still saw recurrence.
"[T]he tests are highly accurate when they are positive, but not as accurate when they are negative," Polsky said.
The study's results were published April 15 in the journal The Lancet Oncology. The next step, Polsky said, is to make the test available to a clinical molecular pathology laboratory, where it can be used to make decisions about treatment. A clinical trial can then show whether using the blood test leads to better outcomes than not using them — a measure called "clinical utility."
"Demonstrating clinical utility of the test would be a major advance for the management of melanoma patients whose disease has spread beyond the skin," Polsky said.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.