Novavax's new COVID vaccine cleared for use by FDA
An updated COVID-19 vaccine made by Novavax has been authorized by the FDA, joining the two vaccines already cleared for use.
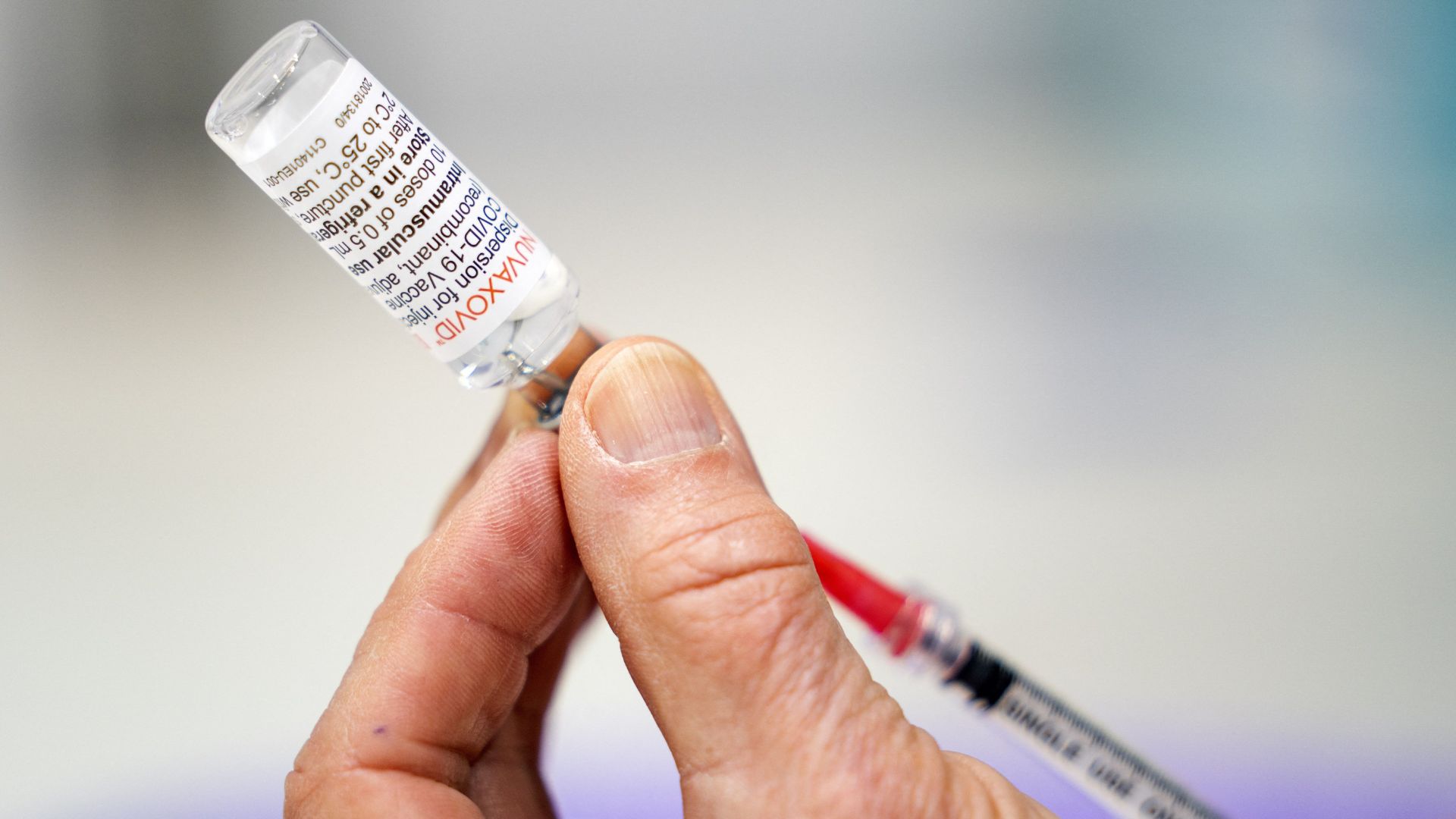
The Food and Drug Administration (FDA) has authorized a new formulation of the COVID-19 vaccine made by the Maryland-based pharmaceutical company Novavax, the agency announced Tuesday (Oct. 3). The newly updated "nanoparticle" vaccine joins the Pfizer and Moderna COVID-19 vaccines already cleared for use in the U.S.
The Novavax shot now includes a spike protein — a pointy structure found on the virus's surface — from a version of the virus that is currently circulating, called XBB.1.5. The widespread subvariant branched off of the omicron family tree. Compared to its old formula, the new Novavax shot is expected to provide better protection against both XBB.1.5 and its close relatives that stem from the same branch.
The shot has been authorized for use in people ages 12 and older.
"Today's authorization provides an additional COVID-19 vaccine option that meets the FDA's standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization," Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in the agency's announcement. "As we head into the fall season and transition into 2024, we strongly encourage those who are eligible to consider receiving an updated COVID-19 vaccine to provide better protection against currently circulating variants."
Related: Who should get the new COVID vaccines? What to know about the 2023-2024 shots
Updated COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved in September, but the Novavax shot is based on different technology.
The Pfizer-BioNTech and Moderna shots contain mRNA, a genetic molecule that carries instructions for human cells to build the spike protein once the vaccine enters the body. In contrast, the Novavax vaccine contains nanoparticles made out of lab-made spike proteins — so the spike proteins are made by cells in a lab, rather than inside the human body.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
In addition, the Novavax shot contains an "adjuvant," a substance that revs up the immune system to mount a stronger response to the vaccine.
The FDA says that individuals who have previously been vaccinated for COVID-19 can get one dose of the updated Novavax shot, provided that their last shot was given at least two months ago. People who have never been vaccinated against COVID-19 can get two doses of Novavax, spaced three weeks apart. People with weakened immune systems can consider getting a second dose of the updated vaccine two months after their first.
Additional doses "may be administered at the discretion of the healthcare provider, taking into consideration the individual's clinical circumstances," the FDA noted.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.