Scientists 'rejuvenate' the aged eggs of mice — is it possible in people?
Researchers have developed a method of reversing the aging process in mouse egg cells.
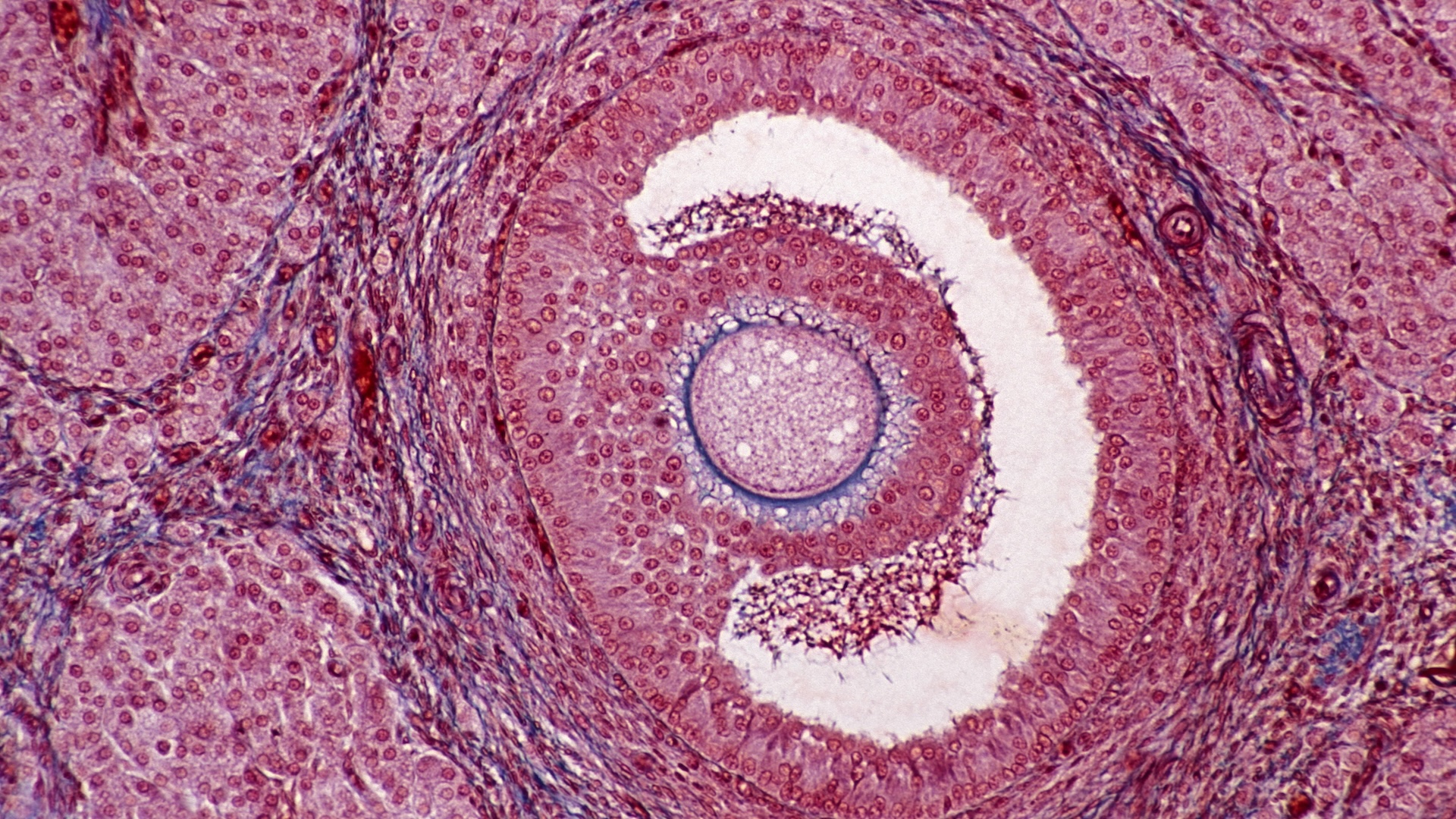
Aging egg cells can be rejuvenated when placed inside young follicles, a new study of mouse cells suggests.
The study could serve as proof-of-concept for future fertility treatments aimed at reversing aging in human egg cells — but much more research is needed to translate these findings to people.
As immature egg cells, called oocytes get older, they begin having problems with cell division. This can result in aneuploidy, in which the chromosomes in the early oocyte don't separate correctly, resulting in extra or missing chromosomes. This causes higher rates of miscarriage.
People can now freeze their oocytes to help preserve their ability to have children. However, there is currently no method of reversing the effects of aging on older oocytes.
Related: Human aging accelerates dramatically at age 44 and 60
In a paper published Monday (Sept. 7) in the journal Nature Aging, scientists at the National University of Singapore demonstrated a new way to model the maturing oocyte in the lab. Through that work, they found that oocytes from older mice that were grown with young mouse cells became rejuvenated, and this improved the rates of live births when the eggs were implanted back into mice.
Senior study author Rong Li, director of the Mechanobiology Institute at the University of Singapore, and her team have been interested in cellular aging for a long time. They started studying the oocyte when they realized that the ovary is the fastest aging organ in the body.
Get the world’s most fascinating discoveries delivered straight to your inbox.
"So when someone is only 40 years of age, everything else is very young and healthy, but the ovary is very old, [limiting] reproductive ability," Li told Live Science. This also makes ovaries a great model for the study of aging, in general.
As an oocyte matures, it undergoes multiple rounds of cell division before it's eventually released from the ovary during ovulation. This complex and energetic process is made possible by a follicle that surrounds the maturing oocyte and provides the energy and nutrients needed through thin filaments called tranzonal projections, or TZPs. Without these lifelines to the follicle, an oocyte cannot mature properly.
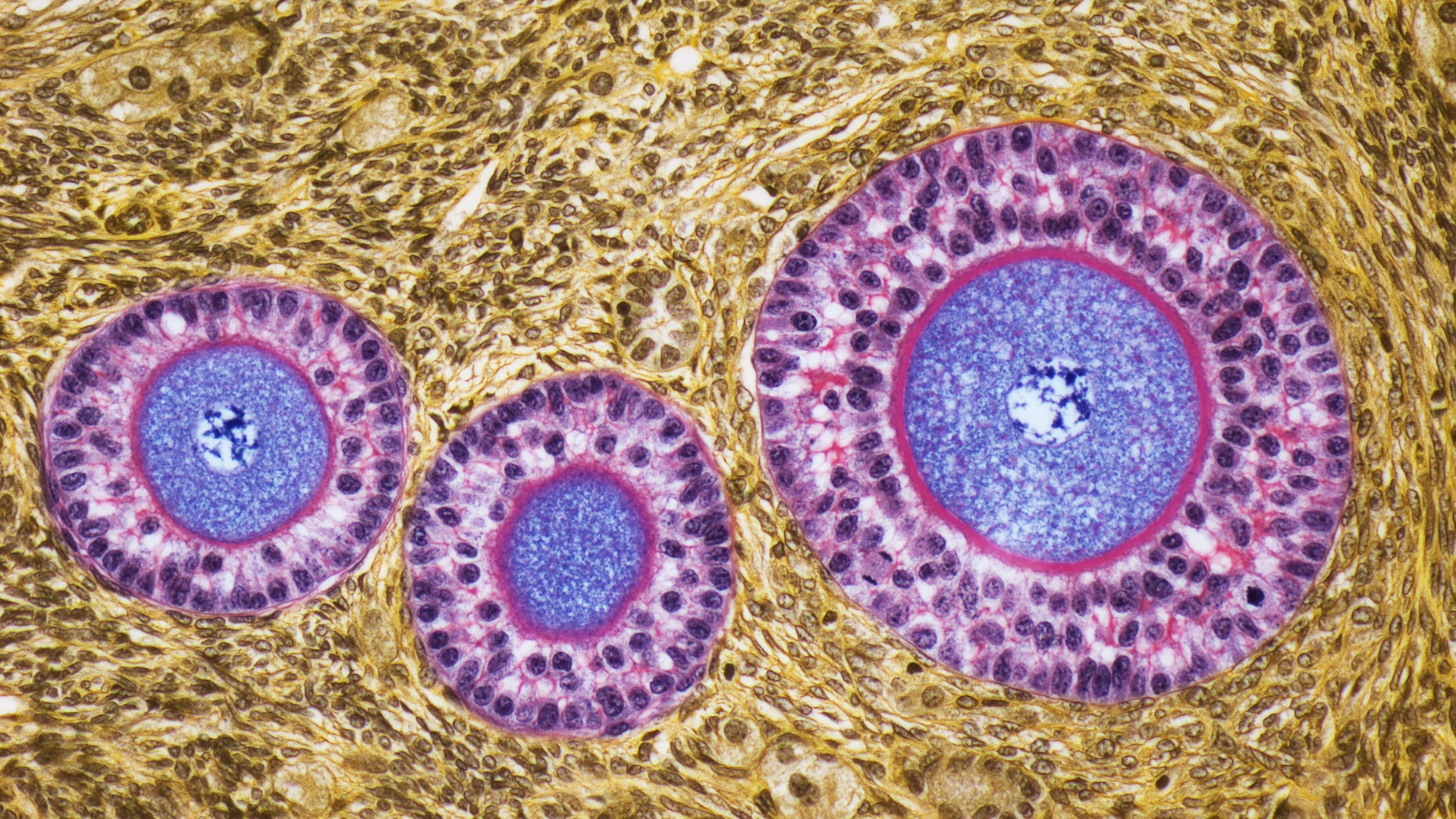
Earlier work on oocyte aging focused on later stages of development, but Li and her team wanted to look earlier when the oocyte is going through the most extreme stages of maturation and cell division that show the most TZP connections.
First author Haiyang Wang, a senior research fellow in the Li lab, designed a 3D system that enables scientists to implant oocytes from one mouse into the empty follicle of another. Wang first removes the oocyte and follicle — which are slightly larger than the width of a human hair — from the mouse ovary by hand using a microscope and tiny tools. When the oocyte is separated from its follicle and then joined to a new one, the TZPs reform and the connections between the follicle and oocyte are reestablished.
Related: Should we rethink our legal definition of a human embryo?
"This is an innovative technology, it's very useful, and it can test the [effect of] environmental factors on oocyte aging," Wang told Live Science.
But Li and her team wanted to go a step further. "If we put an old oocyte into a young [follicle], can it actually become younger?" Li said "Which is also asking another question: is aging reversible?"
The researchers tried implanting oocytes from aging mice into young mouse follicles, finding that the older oocytes showed an uptick in TZP connections, comparable to much younger young egg cells. The eggs also showed improved rates of maturation and about half the rate of chromosomal abnormalities seen in unmodified cells. They also showed quadruple the rate of live births compared to baseline when they were fertilized and reimplanted into mice.
This is the first study to show that oocyte aging can be reversed, and it suggests that it's this connection with younger follicle cells that turns back the clock.
"This impressive study highlights the critical role of the follicular environment in oocyte quality," said Farners Amargant i Riera, a professor of obstetrics and gynecology at Washington University in St. Louis who was not involved in the study. "In addition, it shows that methods to rejuvenate this environment are critical to improve oocyte quality and extend fertility," she told Live Science in an email.
How could these results translate to the problem of age-related fertility? The team would first need to validate the same model and experiments in human cells. But eventually, Li and her colleagues think it would be possible to make a commercial cell line for follicle-forming cells that can be cultured alongside aging oocytes. This, in theory, could be a viable treatment to rejuvenate the old eggs and thus reduce the chance of miscarriage, they propose. Such a treatment would likely be used in the context of IVF.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Jennifer Zieba earned her PhD in human genetics at the University of California, Los Angeles. She is currently a project scientist in the orthopedic surgery department at UCLA where she works on identifying mutations and possible treatments for rare genetic musculoskeletal disorders. Jen enjoys teaching and communicating complex scientific concepts to a wide audience and is a freelance writer for multiple online publications.


