FDA bans red dye No. 3 in food
The FDA will no longer allow red dye No. 3 in foods or ingested drugs, citing evidence that high doses of the dye can cause cancer in male rats. There is no evidence it's carcinogenic in humans.
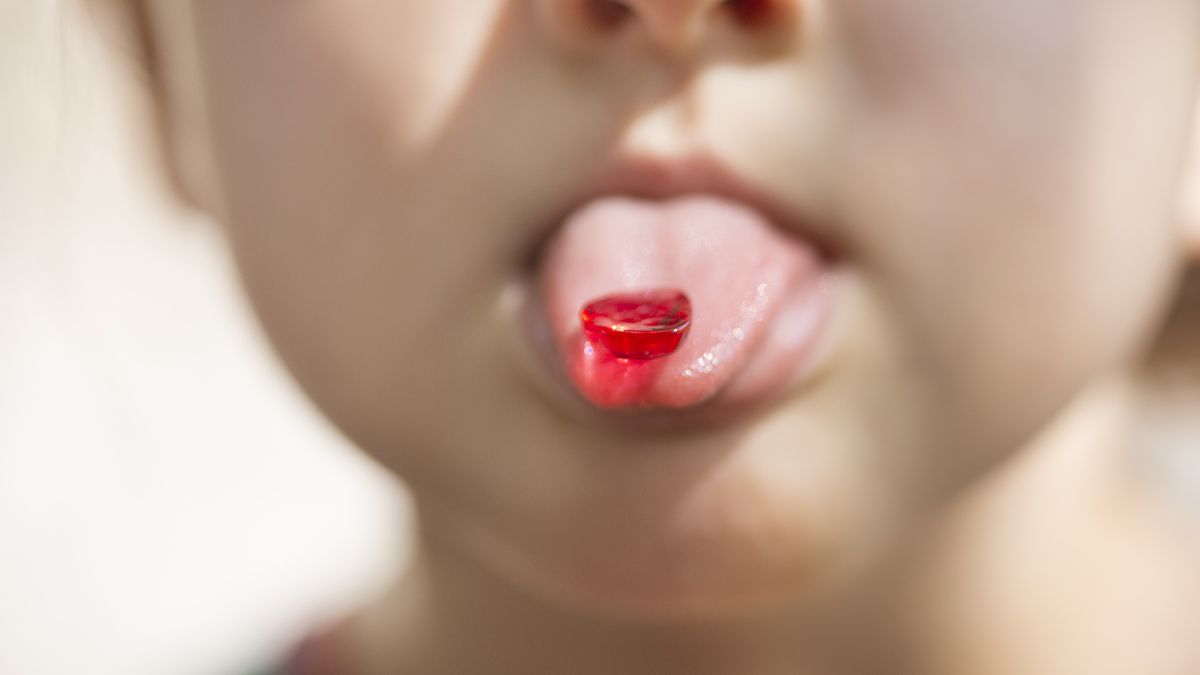
The U.S. Food and Drug Administration (FDA) will no longer allow red dye No. 3 to be used in food, drinks or ingested drugs, like cough syrup, the agency announced Wednesday (Jan. 15).
In its announcement, the FDA cited the Delaney Clause — part of the Federal Food, Drug and Cosmetic Act — as the reason for its decision. This clause requires that the FDA ban food and color additives that are found to cause cancer in humans or animals.
In this case, "or" is the operative word in that clause. In several studies, red dye No. 3 was linked to cancer in male lab rats, but these cancer-causing effects have not been seen in other animals or in humans, the FDA noted.
Nonetheless, those rat studies compelled the FDA to ban the additive from cosmetics and topical drugs back in 1990, The Washington Post reported at the time. That decision had followed a petition that asked the FDA to look into these specific uses for the dye. In the end, the agency also cited the Delaney Clause when removing the additive from cosmetics and topical drugs, and now, it has applied the same logic to foods and ingested drugs.
Related: What is brominated vegetable oil, and why did the FDA ban it in food?
One of the rat studies involved feeding 70 male rats very high doses of red dye No. 3, equivalent to 4% of their diets over a lifetime. Fifteen of the rats developed tumors of the thyroid, a gland in the throat, but most of the tumors were not cancerous. Tumors did not show up in male rats fed lower doses of the dye or in female rats fed any dose. And when scientists did the experiment in mice, rather than rats, they didn't see tumors in males or females.
Later studies suggested that the tumors stemmed from a specific hormonal change triggered by the accumulation of red dye in male rats. But that hormonal mechanism is only relevant to rats, the Post reported.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
In short, "the way that FD&C Red No. 3 causes cancer in male rats does not occur in humans," the FDA emphasized in its announcement. What's more, "relevant exposure levels to FD&C Red No. 3 for humans are typically much lower than those that cause the effects shown in male rats." The available evidence therefore suggests that ingesting red No. 3 does not put humans at risk.
However, "it doesn't matter, because the FDA mandate under the Delaney Clause says that if it shows cancer in animals or humans, they're supposed to keep it from the food supply," Jennifer Pomeranz, an associate professor of public health policy and management at New York University's School of Global Public Health, told CNN.
As such, the FDA has banned the dye "as a matter of law," its statement says.
Red dye no. 3, also known as erythrosine, gives products a bright, cherry-red color. The FDA noted that the dye is found in some candies, cakes, cookies and frozen desserts, among other products, but that it's not as common as other food dyes.
Specific brands of fruit cocktails, lollipop rings, beef sticks and candy corn are among the foods that contain the dye, according to a database compiled by the Environmental Working Group (EWG), a consumer advocacy nonprofit.
The EWG, which has a sister lobbying organization called EWG Action Fund, was a vocal proponent of the red dye No. 3 ban. In 2022, it and other organizations petitioned the FDA to reevaluate the additive, asking the agency to specifically consider the Delaney Clause. (The EWG has historically drawn some criticism for exaggerating the dangers of various chemicals in food and the environment.)
Any U.S. food manufacturers who use red dye No. 3 will have until January 2027 to reformulate their products, while drug manufacturers will have until January 2028. Products imported into the country also must comply with the ban.
Disclaimer
This article is for informational purposes only and is not meant to offer medical advice.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.