CRISPR can treat common form of inherited blindness, early data hint
In a small trial, some people with inherited vision loss experienced improvements in their sight after being treated with CRISPR.
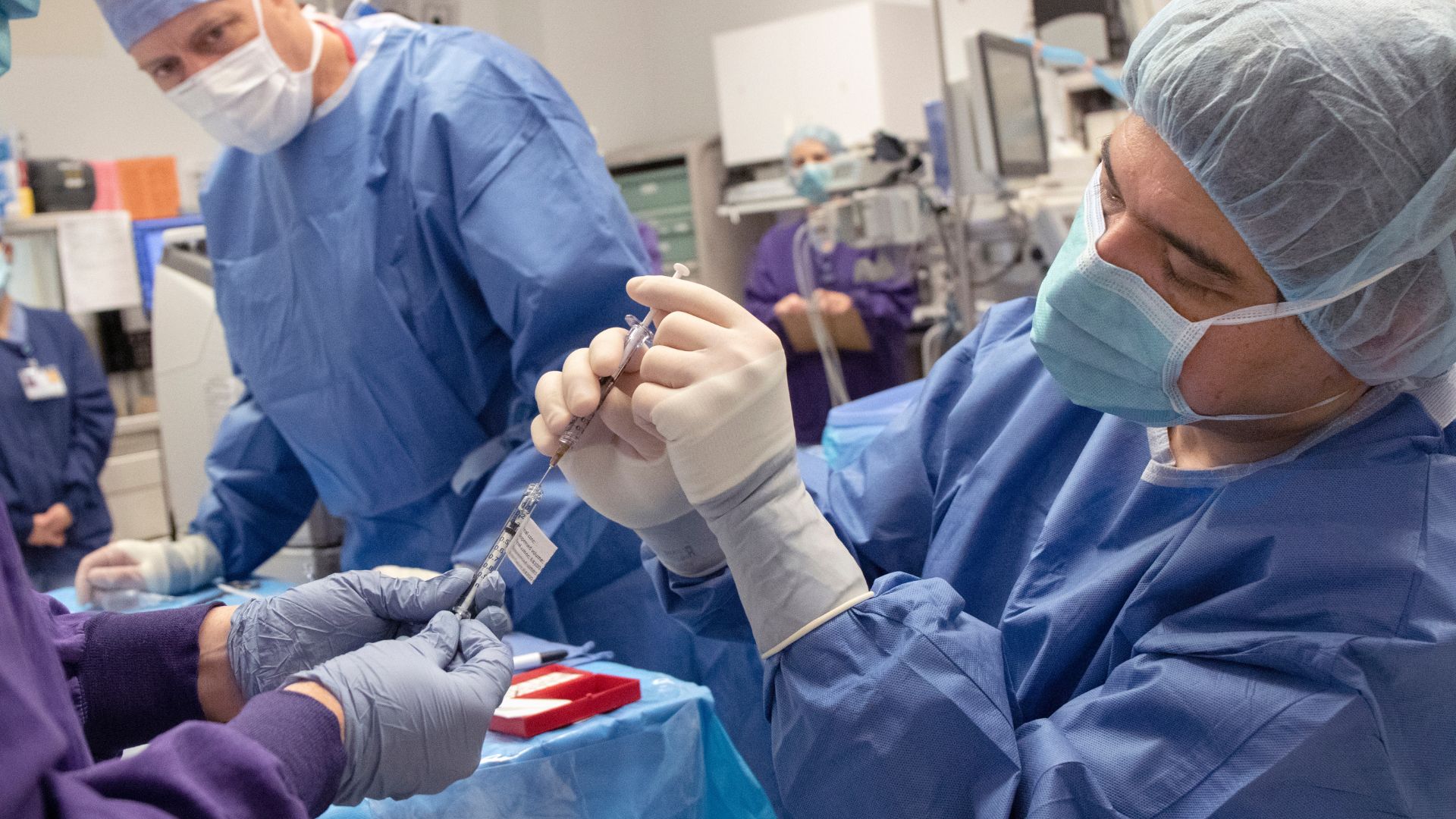
A CRISPR therapy injected directly into the eye shows promise in treating the most common form of inherited vision loss in children, an early trial suggests.
This form of vision loss, called Leber congenital amaurosis (LCA), is often evident at birth and results from the dysfunction or death of light-sensing cells called photoreceptors in the retina, at the back of the eye. Such problems happen due to mutations in any of at least 20 genes.
Some of the most common causes of LCA are mutations in the gene that codes for centrosomal protein 290 (CEP290). More than three-quarters of the people with the disease carry a particular mutation that affects CEP290, which is crucial for photoreceptors to function properly.
LCA currently has no cure — but now, there's evidence that the famous CRISPR gene-editing tool could be safely used to improve the vision of some people with the condition. The results of the early-stage trial were published May 6 in The New England Journal of Medicine.
The results show the promise of using CRISPR to treat inherited eye diseases, Dr. Mark Pennesi, co-author of the report and a researcher at Oregon Health & Science University, told Live Science in an email.
"This is just a start and more work is needed, but the proof of concept is exciting," he said. (Pennesi is a consultant with Editas Medicine, the trial's sponsor.)
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The trial is also notable in that it included the first person to ever receive a CRISPR-based treatment directly into the body. By comparison, the first approved CRISPR therapy involves removing cells from the body, editing them in a lab and then returning them to the patient.
The trial included 14 participants — 12 adults and two children. All carried the specific mutation in the CEP290 gene that affects a majority of LCA patients. The participants received a single injection of the CRISPR treatment, called EDIT-101, into the eye with the most significant vision loss. The other eye served as a comparison.
EDIT-101 contains tiny guides that lead pairs of "molecular scissors" — called Cas9 enzymes — to the mutant gene CEP290. The scissors snip out the faulty portion of the gene, thus restoring its function.
The team used a CRISPR-based strategy because CEP290 is a large gene, making it a difficult target for conventional gene therapy, Pennesi said. Some gene therapies use modified viruses to deliver functional genes into cells, to replace faulty genes, but the CEP290 gene is too large to fit into such a delivery system.
Following this treatment, all of the participants underwent vision tests, which were conducted every three months for one year and then followed by less frequent monitoring for two years. By the end of the trial period, 11 of the 14 volunteers had measurable improvements on at least one vision test, while six experienced improvements in two or more tests. One trial participant shared that they could find their phone after misplacing it and could see the small lights on their coffee machine, which they couldn't do before treatment.
Related: Gene therapies restore hearing in several kids with inherited deafness
Those who didn't show measurable improvements were generally at a more advanced stage of the disease, in which their cells showed a high level of dysfunction at baseline, the trial runners noted. None of the participants experienced adverse side effects of the treatment.
Although EDIT-101 can treat the cells that are present in the retina, it cannot reverse the loss of cells that have already died, Pennesi said. That means participants can experience some improvement in their vision, but it remains decreased, he explained.
"The therapy is not a cure," he said.
The next step would be to test the therapy in a larger number of patients. The team specifically hopes to test the drug in younger patients, "who we hope might have even better outcomes," Pennesi said.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Sneha Khedkar is a biologist-turned-freelance-science-journalist from India. She holds a master's degree in biochemistry and a bachelor's degree in microbiology and biochemistry. After her master's, she worked as a research fellow for four years, studying stem cell biology. Her articles have been published in Scientific American, Knowable Magazine, and Undark, as well as several Indian platforms such as The Hindu and The Wire Science, among others. Besides writing, she enjoys a good cup of tea, reading novels and practicing yoga.









