'More Neanderthal than human': How your health may depend on DNA from our long-lost ancestors
Neanderthals and humans mated millennia ago, and their legacy lives on in us today. Here's how.

The group had traveled for thousands of miles, crossing Africa and the Middle East until finally reaching the dimly lit forests of the new continent. They were long-vanished members of our modern human tribe, and among the first Homo sapiens to enter Europe.
There, these people would likely have encountered their distant cousins: Neanderthals.
These small bands of modern-human relatives had hooded brows, large heads and squat bodies, and they had spent epochs acclimating to Europe's colder climate. At several points across millennia, these two forms of humanity would meet, mingle and mate.
Tens of thousands of years later, these ancient encounters have left traces in the genetic code of billions of humans alive today. The lingering genes affect us in ways large and small, from our appearance to our risk of disease.
"In some places in our genome, we're more Neanderthal than we are human," Joshua Akey, a professor of integrative genomics at Princeton University, told Live Science.
These were our closest human relatives, and this is their legacy.
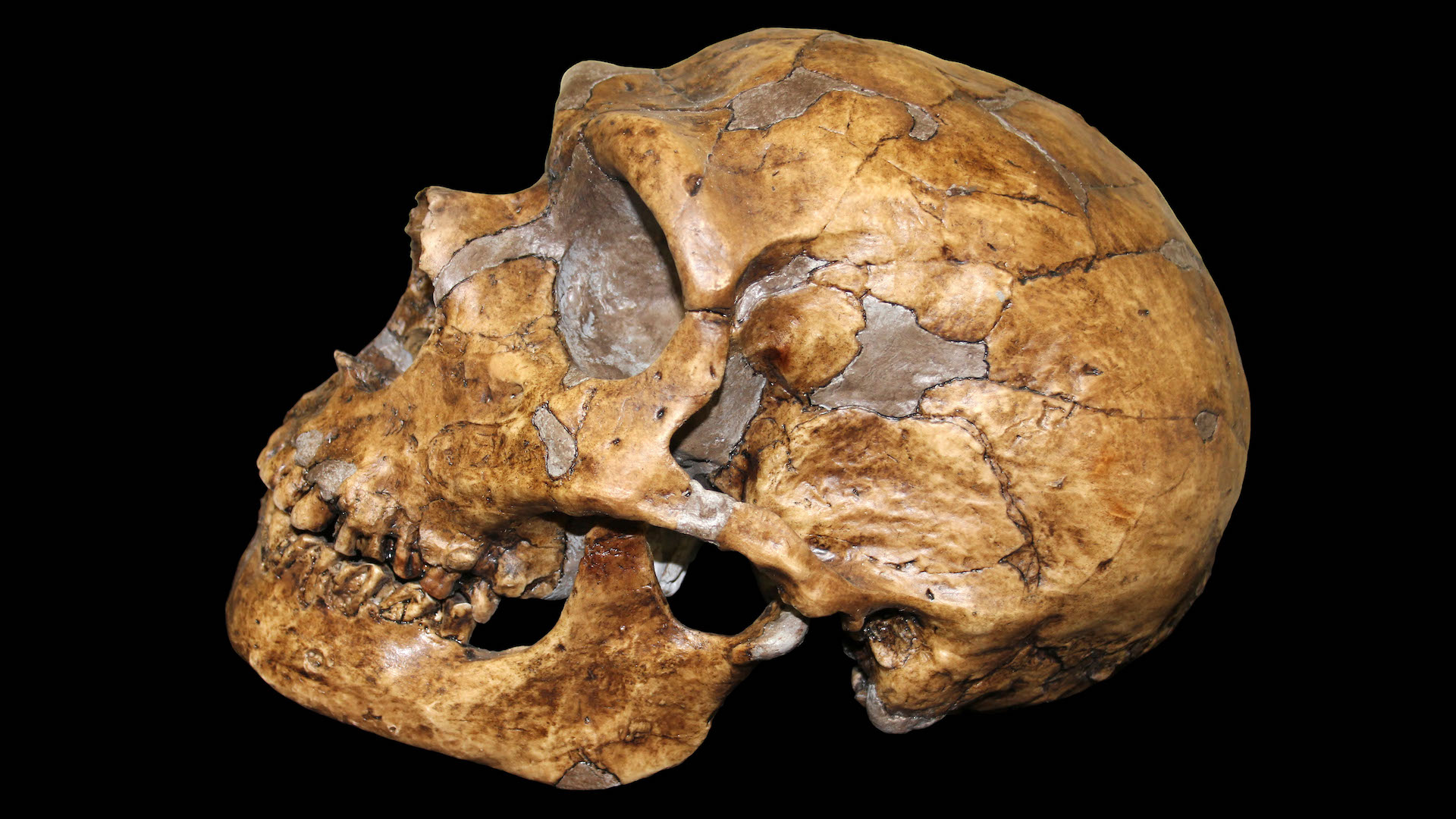
The first encounter
By 75,000 years ago, but possibly up to 250,000 years ago, the ancestors of most modern Eurasians first ventured out of Africa and into Eurasia. Here, modern humans came face-to-face with Neanderthals, who last shared a common ancestor with modern humans hundreds of thousands of years earlier and had been living in these continents ever since. On multiple occasions over the millennia, the groups interbred.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Related: Could Neanderthals talk?
At first, modern humans inherited whole chromosomes from Neanderthals, Sriram Sankararaman, a professor of computer science, human genetics and computational medicine at UCLA, told Live Science. However, from generation to generation, via a process known as genetic recombination, these stretches of DNA were broken up and shuffled around.
Neanderthal DNA was generally "deleterious" to modern humans, meaning it was rapidly weeded out of modern humans' DNA through evolution. This resulted in "deserts of Neanderthal DNA," or large regions of the modern human genome lacking it, Sankararaman said. For instance, scientists think the Y chromosome in males doesn't contain any Neanderthal genes. It may be that genes on the Neanderthal Y were incompatible with other human genes or they may have been randomly lost via a process known as genetic drift.
In people who inherited Neanderthal DNA, the X-chromosome also contains a lot less Neanderthal ancestry than other, non-sex chromosomes carry. This is probably because any harmful or nonfunctional mutations on the X chromosome will be expressed in males, because they lack a matching, functional copy of the gene to compensate. That likely created strong evolutionary pressure to remove such harmful Neanderthal genes from the modern human X, Emilia Huerta-Sanchez, an associate professor of ecology, evolution, and organismal biology at Brown University, told Live Science.
But some Neanderthal DNA helped modern humans survive and reproduce, and thus it has lingered in our genomes. Nowadays, Neanderthal DNA occupies, on average, 2% of the genomes of people outside Africa. However, the frequency of Neanderthal DNA that codes for beneficial traits may be as high as 80% in some regions of the genome, Akey said.

Our physical appearance
For many people, the legacy of Neanderthals is apparent in a highly visible feature: skin color.
A Neanderthal gene variant on chromosome 9 that influences skin color is carried by 70% of Europeans today. Another Neanderthal gene variant, found in most East Asians, regulates keratinocytes, which protect the skin against ultraviolet radiation via a dark pigment called melanin.
Neanderthal gene variants are also associated with a greater risk of sunburn in modern humans. Likewise, around 66% of Europeans carry a Neanderthal allele linked to a heightened risk of childhood sunburn and poor tanning ability.
In some places in our genome, we're more Neanderthal than we are human
Joshua Akey, Princeton University here
Neanderthals had spent millennia at higher latitudes with less direct sun exposure, which is needed for vitamin D production. Therefore, changes to hair and skin biology may have allowed modern humans to quickly capitalize on lower levels of sunlight while still producing enough vitamin D to be healthy, John Capra, an evolutionary geneticist at Vanderbilt University, told Live Science.
"One of the cool things about interbreeding is that instead of waiting for new beneficial mutations to arise, which is a really slow process, you introduce a ton of genetic variation at once," essentially fast-tracking evolution, Huerta-Sanchez said.
Related: What's the difference between Neanderthals and Homo sapiens?
In addition, our ancestors had to adapt to colder Eurasian weather. To do so, they may have acquired Neanderthal genes that affected face shape. In a 2023 study, scientists discovered that modern humans inherited tall-nose genes from Neanderthals. A taller nose may have allowed more cold air to be heated to body temperature in the nose before reaching the lungs, suggested Kaustubh Adhikari, co-senior study author and a statistical geneticist at University College London.
The clock that makes our cells tick
Neanderthal DNA also may have helped H. sapiens adjust to the bigger differences in day and night length at northern latitudes.
Lingering Neanderthal genes affect our circadian clock, which regulates internal processes such as body temperature and metabolism. For instance, some early risers can thank Neanderthals for their circadian clock genes, Capra and colleagues found.
This may have helped our ancestors adapt to shorter winter days farther from the equator, Capra said.
"It seems like it's not that being a morning person is what matters," Capra said. "It's that that's a signal of how essentially flexible your clock is and how able it is to adapt to the variation in light-dark cycles with seasons," he said.
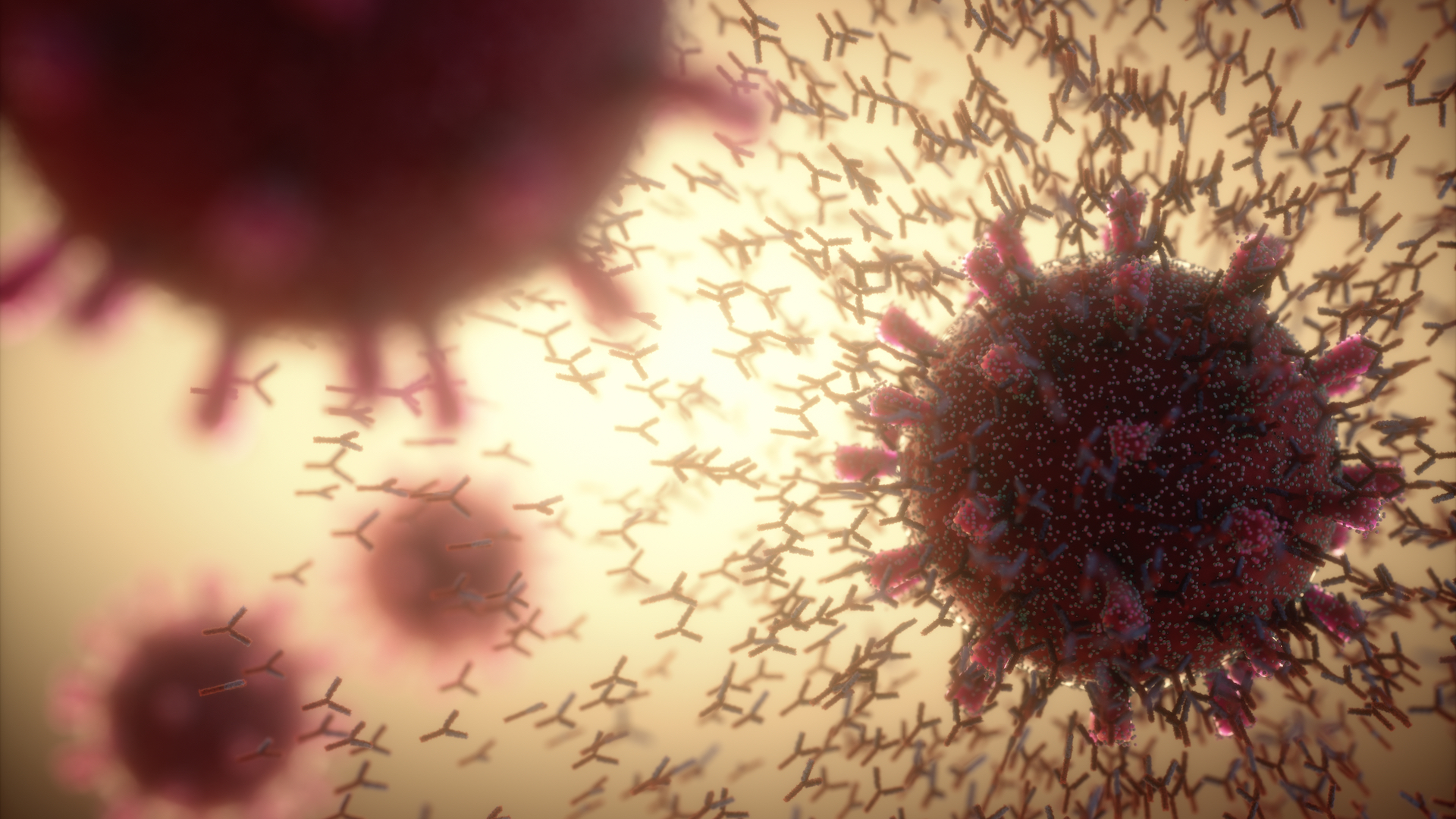
Our internal defenses
Many of the strongly retained Neanderthal genes are tied to immune function.
By the time H. sapiens arrived in Europe, Neanderthals had already spent hundreds of thousands of years fighting infections specific to Eurasia. By mating with Neanderthals, modern humans got an instant infusion of those infection-fighting genes.
"Those pieces of Neanderthal DNA, especially the immune ones, that were already adapted against pathogens that Neanderthals had been living with for a long time started to rise in frequency under natural selection in modern human populations," David Enard, an assistant professor of ecology and evolutionary biology at the University of Arizona, told Live Science.
While many of the ancestral pathogens that sickened ancient humans are lost to time, some of the Neanderthal genes that helped fight them off still work against modern pathogens. For example, a 2018 study by Enard and a colleague revealed that modern humans inherited Neanderthal DNA that helped them combat RNA viruses, a group that today includes the flu (influenza), HIV and hepatitis C.
Related: 10 unexpected ways Neanderthal DNA affects our health
The darker side of Neanderthal DNA
Some of the Neanderthal genes that once helped our ancestors may be harmful in the modern world.
For the most part, Neanderthal genes are not strongly expressed in the brain, which hints that they were strongly selected against during evolution. Neanderthal genes have been linked to mood disorders such as depression and to brain signaling pathways that make people more likely to become addicted to nicotine.
And even the immune boost from Neanderthals may have a downside. In 2016, scientists discovered that Neanderthal genes that prime the immune system to fight pathogens may also predispose people to allergic diseases. In addition, Neanderthal genes have been tied to a higher risk of developing autoimmune diseases, such as Graves' disease, caused by an overactive thyroid; and rheumatoid arthritis, which inflames the joints and even "Viking disease," in which one or more fingers become bent or frozen.
One Neanderthal gene variant may have made us more likely to have a severe case of COVID-19. That variant, found on chromosome 3, is found in half of South Asians and one-sixth of Europeans. But even there, the picture is complicated, as other Neanderthal genes, carried by up to half of people in Eurasia and the Americas, are associated with a reduced risk of severe COVID-19.
"Unfortunately, there are no diseases we can really say, or even traits in general, we can say, 'Oh, you can blame your Neanderthal DNA for that,'" Capra said.
That's especially true for some of the biggest health ailments, such as heart disease and cancer, where dozens or hundreds of genes, along with myriad environmental factors, affect your risk of disease.
What lies ahead
So how long will the traces of these long-lost humans linger in our genomes? Over hundreds of thousands of years, some of these Neanderthal fragments will gradually be eliminated from our genomes. Others will become firmly embedded, Akey said.
In the meantime, there's still much more to learn about how Neanderthals left their mark on us.
"Being able to leverage new genomic technology like CRISPR and gene editing is going to play an important role in understanding the actual underlying biology of how Neanderthal sequences contribute to human traits and diseases," Akey said.
Deciphering what these genes actually do could aid the development of treatments for certain conditions, he said.
And the gene flow wasn't one-way; scientists are also trying to determine how modern-human DNA may have influenced Neanderthals and are applying artificial intelligence (AI) methods to ancient genomes to create a more detailed picture of what our long-lost cousins were like.
Figuring out the role of Neanderthal DNA in our genomes does more than help us understand our health. These bits of DNA can provide clues as to what makes us unique, Sankararaman said.
"Neanderthal DNA entered our genomes at an important time in our history," Sankararaman said, when our ancestors were moving into new environments.
"By looking at the fate of these bits of DNA," he said, "we can hope to understand what were the functionally important regions in our genome over this period of time."

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.


