In a 1st, scientists grow human kidneys inside developing pig embryos
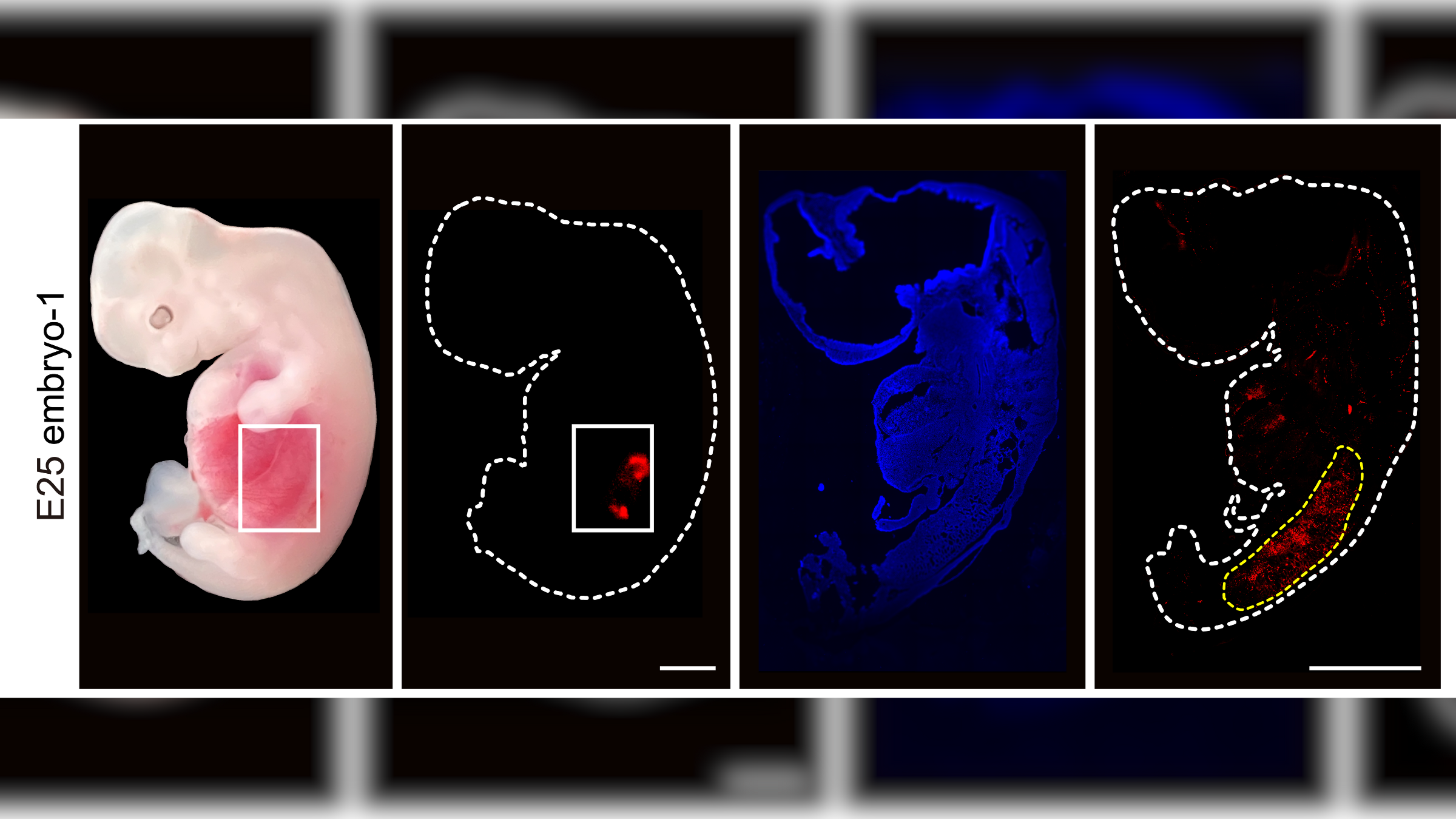
Scientists grew early-stage human kidneys inside pig embryos and found the kidneys were "structurally normal" and made up of around 60% human cells.
Scientists have successfully grown a human organ inside another animal for the first time.
In a new study, published Thursday (Sept. 7) in the journal Cell Stem Cell, researchers inserted human stem cells into genetically tweaked pig embryos. When these were implanted into surrogate pig mothers, the embryos developed early-stage human kidneys within about 28 days.
The research is still in its infancy, but the authors say this technology could one day help relieve the shortage of human organs needed for transplantation.
"Rat organs have been produced in mice, and mouse organs have been produced in rats, but previous attempts to grow human organs in pigs have not succeeded," Liangxue Lai, senior study author and a principal investigator at the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences and Wuyi University, said in a statement.
In previous experiments, scientists harvested pig kidneys and hearts from genetically modified swine and transplanted them into brain-dead organ donors, but this strategy comes with a high risk that the human body would reject the pig organs. The new research aims to limit that problem.
"Our approach improves the integration of human cells into recipient tissues and allows us to grow human organs in pigs," Lai said.
Related: Most advanced lab-made human embryo models look like the real thing
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The kidneys are two bean-shaped organs located either side of the spine, below the ribcage, that filter the blood and remove waste and excess water via urine. They're among the most commonly transplanted organs, but a lack of available kidneys means there's a growing list of people who need one — for instance, around 100,000 people in the U.S. were on the waiting list to receive a kidney in 2020, but only 23,000 received one.
One solution is to integrate human stem cells that can develop into any type of cell, called induced pluripotent stem cells (iPSCs), into the embryos of other mammals to create "mixed," or "chimeric" embryos that can grow human organs. Pigs are a good choice for this because their organs are similar to ours and so is their embryonic development. However, the challenge is that pig cells in the embryo can easily outcompete the human cells and require different nutrients and chemical signals to grow.
In the new study, researchers overcame these challenges by using CRISPR technology to disable two genes that would have normally enabled embryonic pig cells to develop kidneys. This created a "niche" for human iPSCs to fill. The researchers also manipulated human iPSCs so that they were more likely to integrate with pig cells by matching their developmental stage. Without this adjustment, the human cells would have been developmentally ahead of their pig counterparts, Science reported.
The team implanted 1,820 of the chimeric embryos into 13 surrogate pig mothers and then terminated the pregnancies and extracted the embryos about a month later. Of these, five embryos contained early-stage kidneys that were made up of around 50% to 60% human cells and were "structurally normal" for this stage of development. They contained cells that would eventually become ureters, the tube-like structures that connect the kidney to the bladder.
Related: How long can organs stay outside the body before being transplanted?
Importantly, the scientists confirmed that the human cells in the embryo were mainly in the kidneys, rather than in other tissues of the embryos, such as sex or nerve cells that could raise ethical questions if they were allowed to reach maturity in baby pigs.
This technology is still a while off being applied to human organ transplantation. One crucial hurdle is the issue of immune rejection, as the kidneys the team created still contained pig-derived cells, such as those that make up blood vessels. A high number of pig embryos also ended up degenerating in the study, so the process's efficiency will need to be addressed with future research.
In the meantime, the team hopes that the findings could improve our understanding of human organ development and developmental diseases.
"Before we get to that late state of making organs that can be on the shelf for clinical practice, this method provides a window for studying human development," Miguel Esteban, study co-author and a principal investigator at the Guangzhou Institutes of Biomedicine and Health, said in the statement. "You can trace the human cells you're injecting and manipulate them so that you can study diseases and how cell lineages are formed."

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30. (emily.cooke@futurenet.com)










