1st gene therapies for sickle cell cleared by FDA, including CRISPR treatment
The FDA approved two new therapies for sickle-cell disease, including the world's first-ever approved CRISPR therapy.
In a historic first, the Food and Drug Administration (FDA) has approved America's first gene therapies for sickle-cell disease (SCD), one of which uses the gene-editing tool CRISPR.
"Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need [for better, long-lasting treatments], and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today," Dr. Nicole Verdun, director of the Office of Therapeutic Products within the FDA's Center for Biologics Evaluation and Research, said in a statement released Friday (Dec. 8).
"Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited," she said.
The U.K. became the first country to approve the CRISPR-based therapy, called Casgevy, in mid-November. Experts anticipated that the FDA would soon echo the decision made by U.K. regulators, as advisors to the FDA had deemed the treatment safe for clinical use back in October.
Related: The world's 1st CRISPR therapy has just been approved. Here's everything you need to know
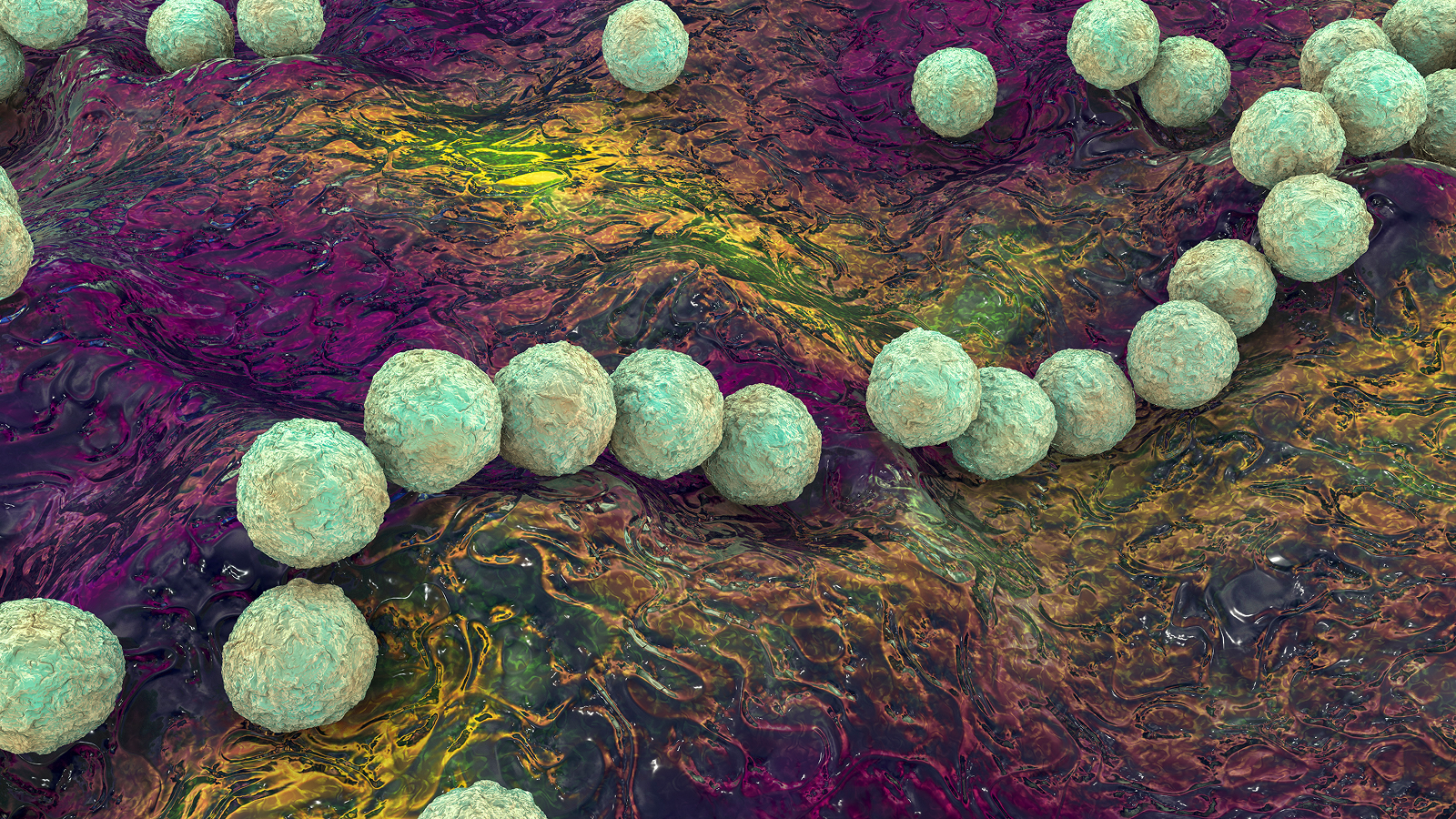
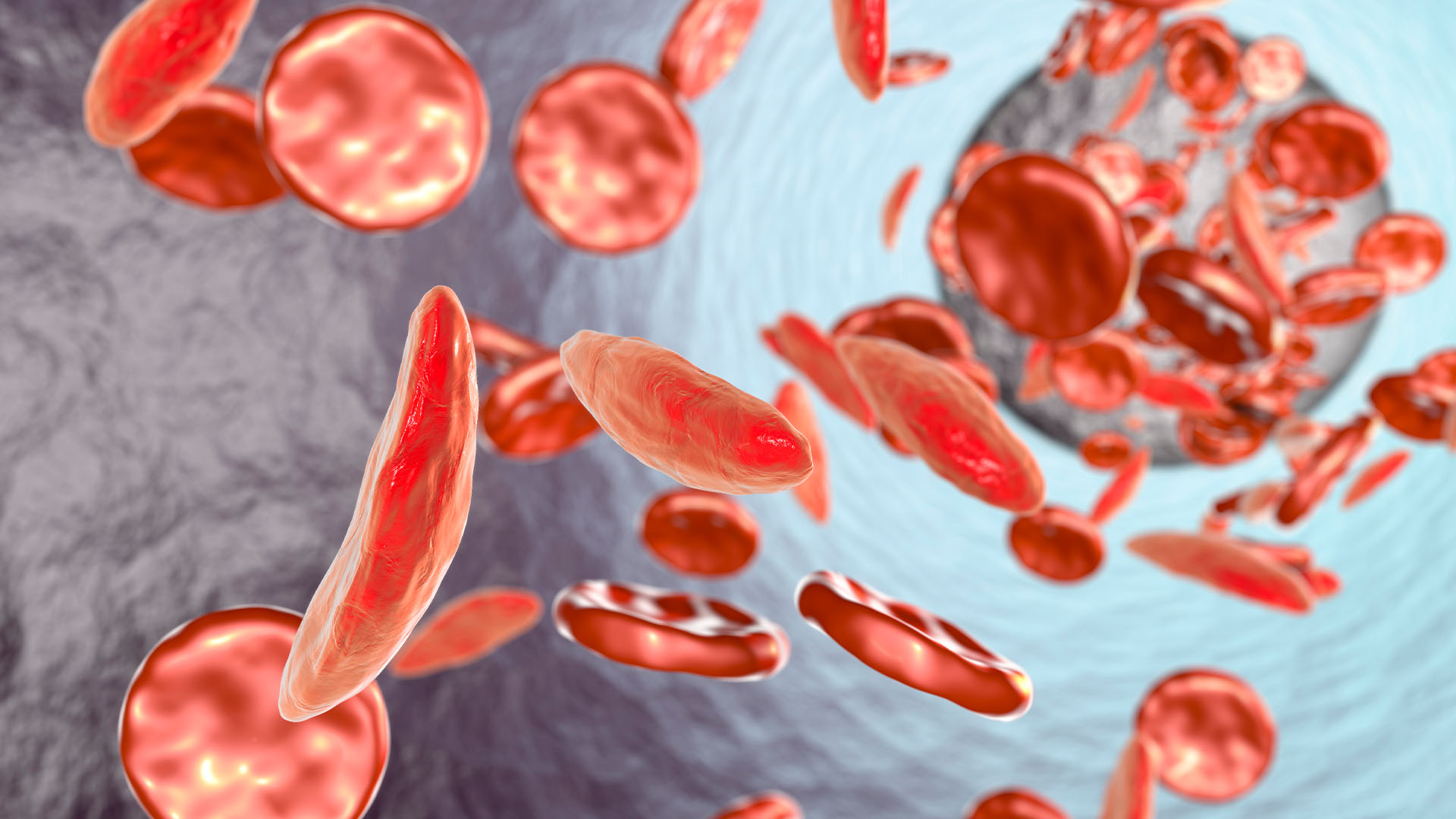
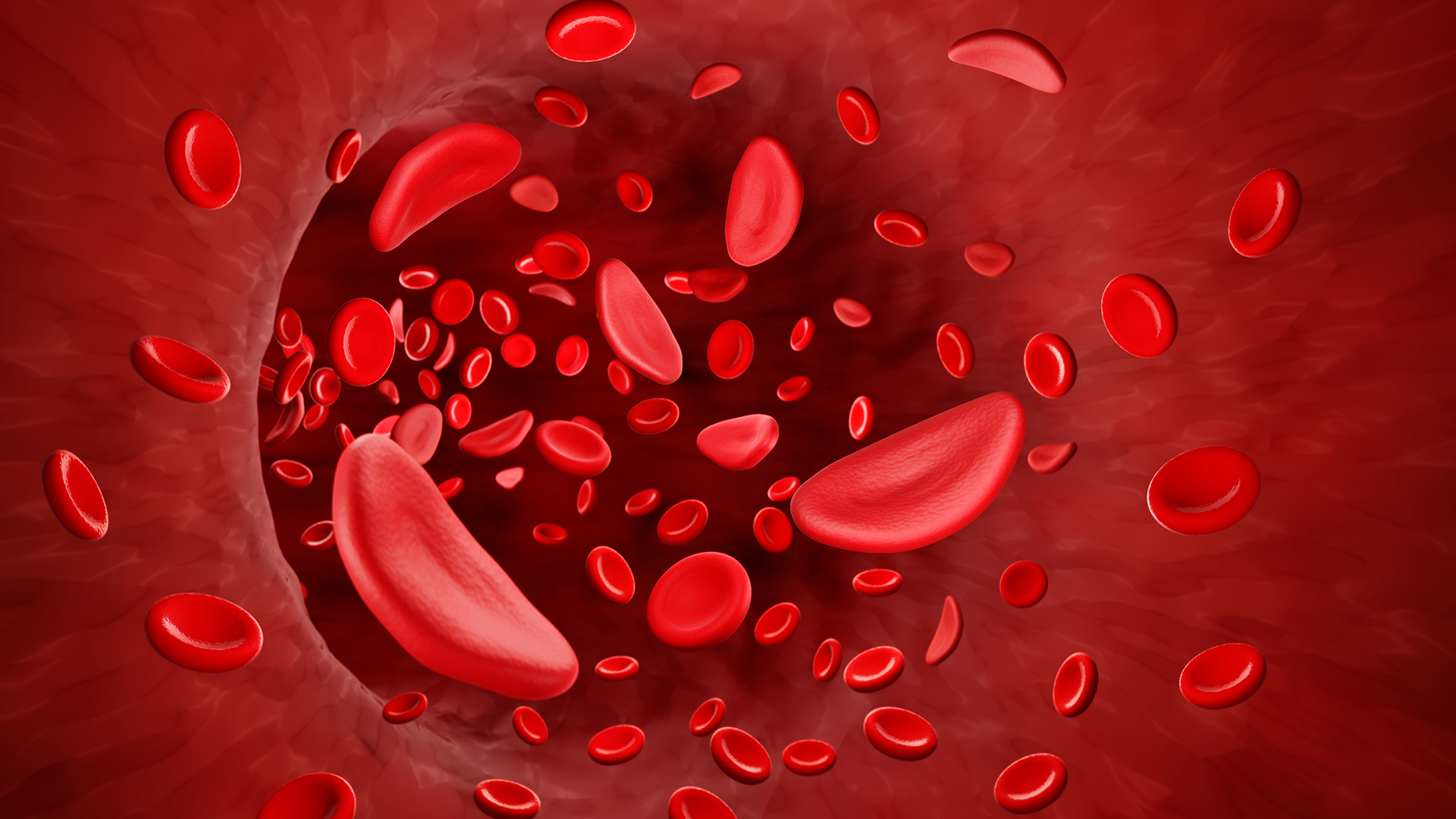
SCD is caused by genetic mutations that change the shape of the protein hemoglobin, which carries oxygen in red blood cells. Red blood cells then become sickle-shaped, rather than round, which causes them to die off quickly and also stick together, blocking blood vessels.
The CRISPR-based therapy Casgevy stops the sickling of cells by switching off a gene called BCL11A.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The CRISPR system can precisely guide a pair of molecular scissors to the gene doctors want to disable and then cut that gene out of a person's DNA. Disabling the BCL11A gene makes it so a patient's cells can make a version of hemoglobin normally made only in the womb. Only the adult version of hemoglobin is affected in SCD, so enabling the body to make this fetal hemoglobin reverses the patient's anemia.
"In patients with sickle cell disease, increased levels of HbF [fetal hemoglobin] prevent the sickling of red blood cells," the FDA stated.
To apply the treatment, doctors first draw a patient's blood stem cells — unspecialized cells that can transform into different cells in the blood. They then edit the cells to disable the BCL11A gene and return them to the patient's body. Before the infusion, the patient must take a chemotherapy drug to eliminate the unedited stem cells still in their bone marrow.
The second gene therapy approved by the FDA, called Lyfgenia, does not use CRISPR. Instead, the treatment uses a harmless virus, called a lentiviral vector, to deliver new DNA into patients' blood stem cells.
The virus inserts a functional hemoglobin gene to replace patients' mutant one. The functional gene makes a version of hemoglobin that's very similar to that seen in adults without SCD, but it has additional properties that help limit the sickling of blood cells. Blood cells change shape in SCD when the abnormal hemoglobin clumps together, or "polymerizes," to form stiff chains within the cells. The tweaked hemoglobin used in Lyfgenia is more resistant to that polymerization than normal hemoglobin, due to its structure.
As with Casgevy, patients take a chemotherapy drug before receiving their new cells treated with Lyfgenia.
Casgevy is approved for SCD patients ages 12 and older with "recurrent vaso-occlusive crises," meaning events where sickled red blood cells block the oxygen flow into organs, causing tissue damage and severe pain. Lyfgenia is approved for patients ages 12 and older with a history of vaso-occlusive events, the broader category of complications that crises fall under.
"Today's actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDA's commitment to facilitating development of safe and effective treatments for conditions with severe impacts on human health," Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
What are mRNA vaccines, and how do they work?
Deadly motor-neuron disease treated in the womb in world 1st