1st-of-its-kind menopause drug targets brain misfiring behind hot flashes
The U.S. Food and Drug Administration approved a new pill for moderate to severe hot flashes.
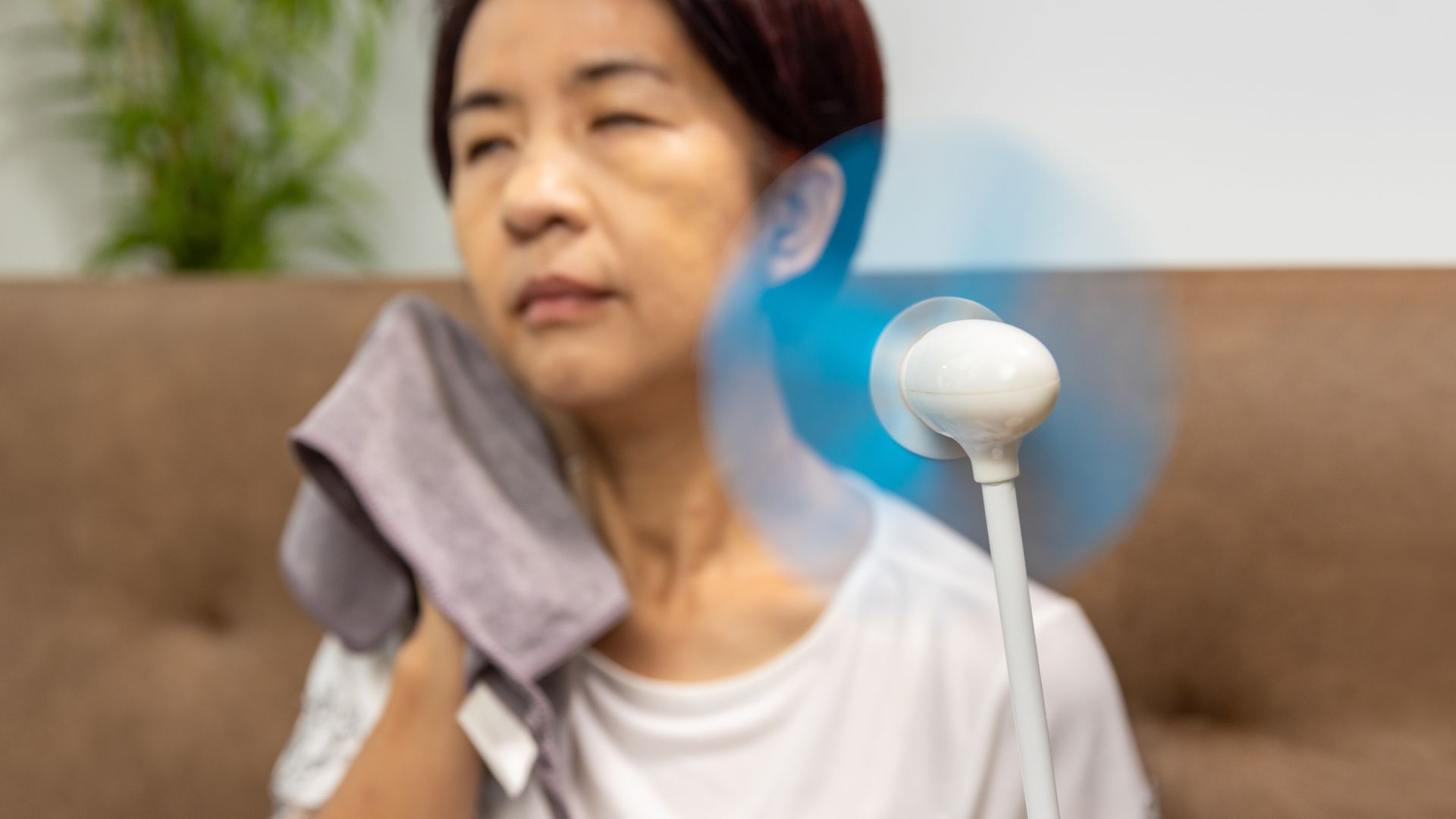
A newly approved drug treats severe hot flashes caused by menopause by blocking specific signals in the brain, rather than using hormones like estrogen, the U.S. Food and Drug Administration (FDA) announced Friday (May 12).
The pill, called Veozah (generic name fezolinetant), is the first of its kind approved to treat menopausal hot flashes. Known as a "neurokinin 3 (NK3) receptor antagonist," the drug effectively blocks NK3 receptors found in a part of the brain called the hypothalamus, an almond-size structure that regulates the body's hormone production and temperature, among other functions.
Why block NK3 receptors? Evidence suggests that, in menopause, these receptors trigger an inappropriate reaction where the body tries to cool itself off when it is not overheated, according to BrainFacts. This results in the flushing and sweating characteristic of a hot flash.
During menopause — defined as the time when one full year has passed since a person's final period — the body produces less and less estrogen as the follicles in the ovaries that once made the hormone steadily decline in number. To make up for this estrogen loss, the brain tries to signal the ovaries to make more of the hormone, and that message is initially sent from the hypothalamus.
Related: Do animals have menopause?
The problem is that the same cells in the hypothalamus that boost estrogen also link up to nearby cells that control body temperature. By releasing a substance called neurokinin B, these cells end up switching on NK3 receptors and triggering hot flashes, according to BrainFacts.
In clinical trials involving about 3,000 people, Veozah significantly reduced the severity and frequency of hot flashes by blocking patients' NK3 receptors. Current data suggests the pill works better than paroxetine, an antidepressant that has also been approved for the treatment of hot flashes and is thought to help regulate hot flash-triggering chemical messengers in the brain, The New York Times reported.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
However, Veozah hasn't been directly compared to hormone therapies, in which estrogen and sometimes progesterone are given to replace the body's dwindling supply. Those are estimated to reduce hot flash frequency by about 75%, according to The New York Times.
Not everyone with hot flashes is recommended to use these hormonal treatments, though, because the therapies have been linked to an increased risk of blood clots, heart attacks, strokes and breast cancer, and people with a history of certain medical conditions face a particularly high risk of such adverse effects, according to the FDA.
An estimated 75% of people have hot flashes during menopause. Hot flashes often begin before the final menstrual period and typically continue for two years or less after onset, according to Johns Hopkins Medicine.
Veozah carries a potential risk of liver injury. In the clinical trials, 25 patients who took the drug developed "elevated hepatic transaminases," a sign of liver injury, compared with eight people in the control groups. Thus, patients with existing liver scarring, damage or disease should not take Veozah, the FDA advises, and those who do take it should be screened for signs of liver damage in their blood work every three months for the first nine months of using the medication.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.