Antibiotic resistance makes once-lifesaving drugs useless. Could we reverse it?
Evolutionary biologist Tiffany Taylor explores the work being done to resensitize antibiotic-resistant bacteria to drugs as a strategy to defend ourselves against the growing antibiotic resistance crisis.
The world is facing an ever-increasing threat from bacteria evolving resistance to known antibiotics, rendering the essential drugs ineffective. But now, researchers are exploring promising new treatment strategies, with the aim of making those resistant bacteria susceptible to drugs once more.
The rise of antibiotic-resistant bacteria has been dubbed the "silent pandemic" due to its stealthy global spread and lack of urgent public attention, in comparison to other pandemics such as COVID-19, especially in regions where antibiotic use remains largely unchecked. Estimates from a 2019 report published by the Centers for Disease Control and Prevention (CDC) suggest that resistant bacteria killed at least 1.27 million people worldwide that year, with 35,000 of those deaths occurring in the U.S. alone. That marked a 52% increase in U.S. deaths from resistant microbes since the CDC's previous report in 2013.
"Antibiotic resistance is a major public health threat because so much of modern medical care depends on antibiotics — childbirth, cancer treatment, transplants, operations, and infections," Zamin Iqbal, a professor of algorithmic and microbial genomics at the University of Bath in the U.K., told Live Science in an email.
What's causing this mounting disaster? The overuse and misuse of antibiotics in both medicine and agriculture are the major culprits.
Related: Superbugs are on the rise. How can we prevent antibiotics from becoming obsolete?
That's because antibiotic resistance arises from a natural evolutionary process — one in which the fittest bacteria with the right tools to outcompete an antibiotic survive to pass on those tools.
When a population of bacteria is exposed to an antibiotic, any genetic mutations that allow the bacteria to survive the drug will quickly spread between bacterial cells. Repeatedly using different antibiotics can lead bacteria to develop resistance to multiple drugs, resulting in strains that are no longer treatable with any known antibiotics — with potentially fatal consequences.
Get the world’s most fascinating discoveries delivered straight to your inbox.
In light of this chilling reality, we must prolong the effectiveness of the antibiotics we already have as long as possible as new, alternative solutions are devised in the background. One way to achieve this is by finding strategies that can reverse the process by which bacteria become resistant, pushing them back into a drug-sensitive state.
To achieve this, Joana Azeredo, an associate professor at the University of Minho in Portugal, exploits a natural enemy of bacteria: bacteriophages, or viruses that infect bacteria. Known as phages for short, these viruses are often discussed as a promising treatment strategy against antibiotic-resistant bacteria because of their potential to kill the cells they infect. However, rather than killing the bacteria, the phages Azeredo is interested in insert themselves into the bacteria's genomes.
Her research uses genetically engineered phages as "Trojan horses" to deliver genes that ultimately make bacteria vulnerable to antibiotics by eliminating the resistance genes they carry.
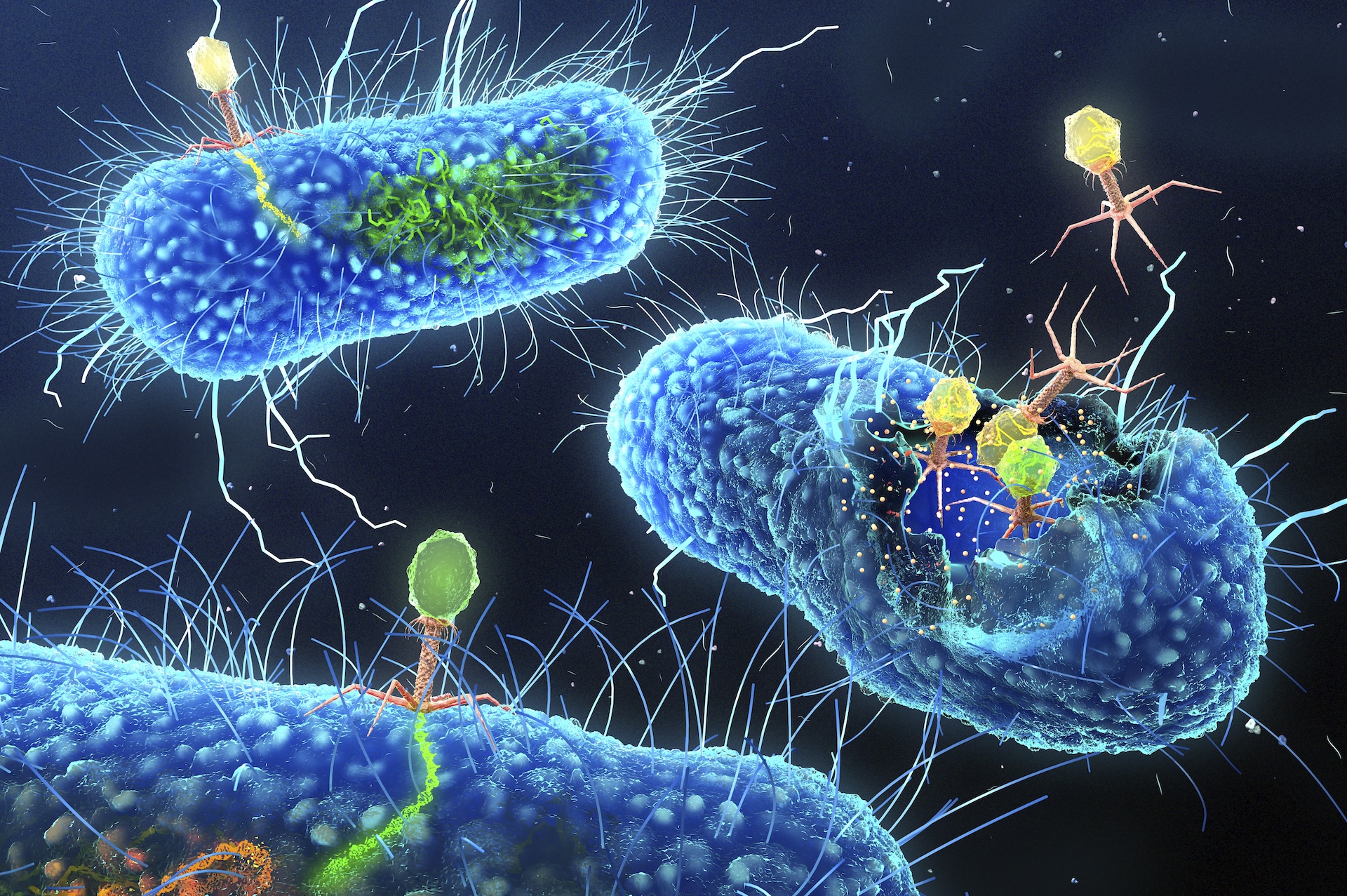
A common resistance mechanism bacteria use against both phages and antibiotics are biofilms, which shield the bacterial cells from harm. Fredrik Almqvist, a professor of organic chemistry at Umeå University in Sweden, together with molecular biologist Christina Stallings, a professor of molecular microbiology at Washington University, are developing chemical compounds that break down the biofilms of drug-resistant bacteria, effectively resensitizing them to antibiotics.
Almqvist and Stallings' research has uncovered a small molecule that disrupts the genetic pathways that allow microbes to form biofilms. The molecule not only blocks this resistance mechanism from evolving in the first place but also restores antibiotic sensitivity to bacteria that have already evolved to use it.
Other researchers are taking a different approach: They're targeting the downstream mechanism of resistance, rather than eliminating its root genetic cause. For example, research from Despoina Mavridou, an assistant professor at the University of Texas at Austin, aims to resensitize resistant bacteria by stopping cells from making a protein that helps fold other proteins.
Folding is a key step that enables a newly made protein to execute a particular function. Mavridou's approach prevents the folding of proteins that enable the bacteria to resist antibiotics. In studies, inhibiting this folding-assistant protein restored the sensitivity of multidrug-resistant bacteria. The inhibitors used in the study have not yet been approved for human use, though, so further research is needed to bring these discoveries to the clinic.
Designing new antibiotics is expensive and difficult, which is one of the many reasons so few are in development. Thus, it's crucial to protect and extend the effectiveness of the antibiotics we already have. The future of the antibiotic resistance crisis is uncertain, but ongoing research offers hope for innovative strategies that could change the course of this global challenge. It is imperative, however, that we learn from previous mistakes. Any new strategies we adopt should anticipate the ways in which bacteria could evolve to resist the treatments.
"It's important to understand how bacteria respond to the selection pressure imposed by antibiotics," Andrew Preston, a professor in microbial pathogenicity at the University of Bath and the editor-in-chief of the journal Microbiology, told Live Science in an email. "We will have some novel treatment strategies come through the pipeline, so it's imperative we consider how we might mitigate/reduce the selection for resistance to prolong their use."
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Tiffany Taylor worked at Live Science in the summer of 2024 as a Fellow of the Association of British Science Writers. She is a professor of Microbial Ecology and Evolution at the University of Bath in the U.K., where her research group studies evolution in real-time in the lab, using bacteria to explore how genes and genomes evolve. She has also authored three children’s books on evolution and genetics. When she is not doing research, she’s usually running – sometimes for pleasure, more often after her two small children.


