Drug could reduce need for insulin in type 1 diabetes, early trial hints
An early trial suggests that a drug commonly used for rheumatoid arthritis could reduce type 1 diabetics' reliance on insulin, but questions remain.
A drug commonly used to treat the autoimmune disease rheumatoid arthritis (RA) could also slow the progression of type 1 diabetes, an early clinical trial suggests.
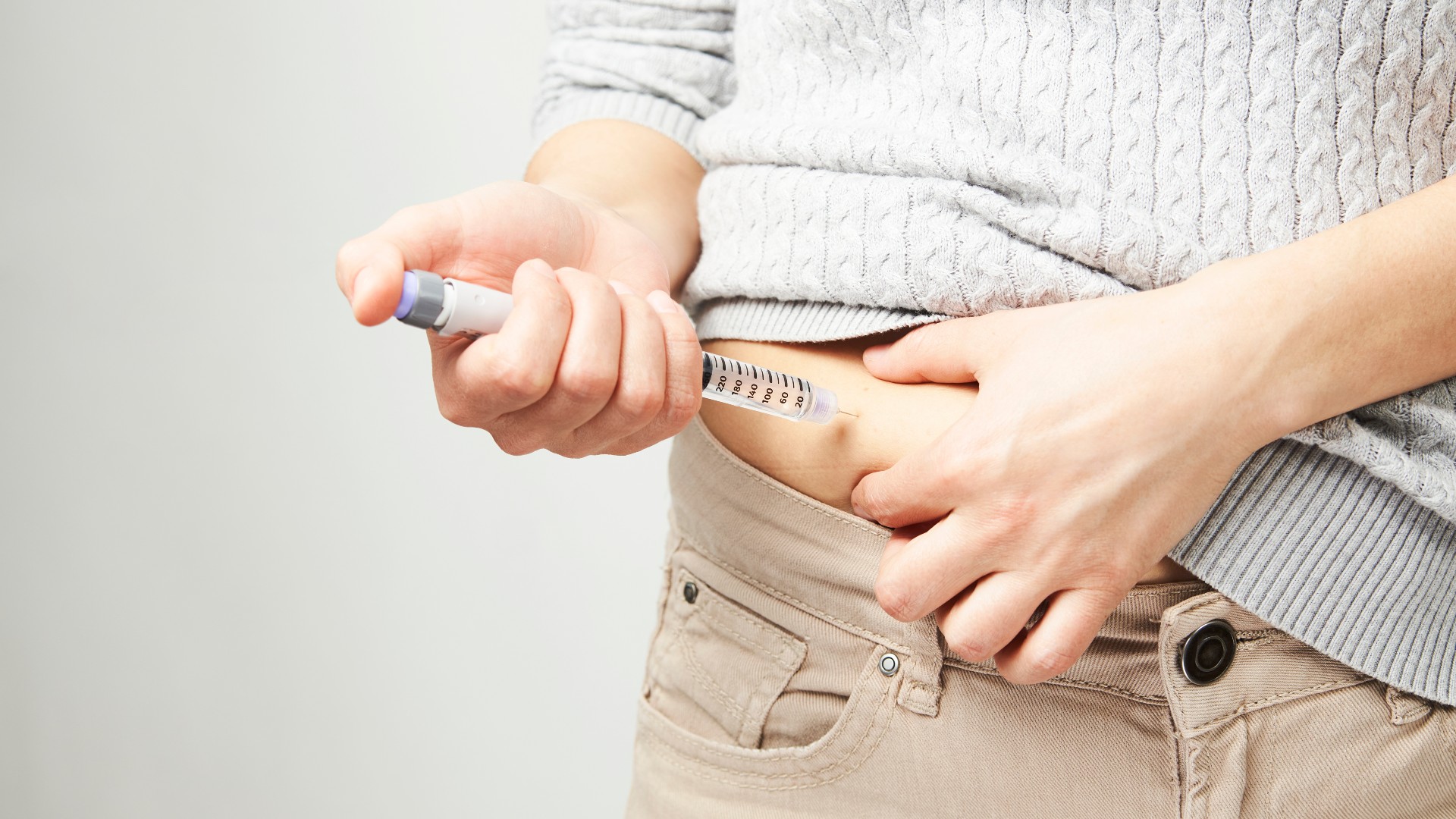
Similar to RA, type 1 diabetes is an autoimmune condition — in RA, the body's immune cells attack the joints, while in diabetes, they destroy insulin-producing beta cells in the pancreas. The resulting insulin deficiency means cells can't remove glucose from the bloodstream, causing blood sugar to skyrocket. Treating type 1 diabetes requires daily insulin injections.
Now, a clinical trial has shown that a tablet prescribed for RA, called baricitinib, may reduce type 1 diabetes patients' dependence on external insulin. The mid-stage trial, published Dec. 7 in the New England Journal of Medicine, found that baricitinib slowed the progression of diabetes in newly diagnosed patients by preserving their body's ability to make insulin.
The motivation behind the research was "to prevent the loss of insulin secretion rather than managing the absence of naturally produced insulin," Helen Thomas, the preclinical lead on the trial and head of the Immunology and Diabetes Unit at Australia's St Vincent's Institute of Medical Research, told Live Science in an email.
Related: 1st ever drug to delay type 1 diabetes approved by FDA
The team enrolled 91 patients ages 10 to 30 who had been diagnosed with type 1 diabetes less than 100 days before the trial began. (At this point after diagnosis, people's bodies still make some insulin.) Of these, 60 patients received 4 milligrams of baricitinib once a day, while the remaining 31 took a placebo pill. Both groups were treated for 11 months.
Throughout the treatment period, the researchers observed no unwanted side events tied to baricitinib, which suggests the drug is safe for people with diabetes.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
After the treatment period, the patients underwent a blood test. Baricitinib-treated patients had higher levels of C-peptide, an indicator of insulin levels, than placebo-treated patients. The pancreas makes C-peptide as it makes insulin, so higher C-peptide level signifies better beta cell function in the baricitinib group.
Indeed, by the end of the study, three baricitinib-treated patients didn't need any external insulin, while others in the group were able to decrease their doses, requiring lesser amounts over time. Meanwhile, the untreated group saw their injected insulin needs slowly increase.
The authors hypothesize that starting patients on baricitinib earlier — immediately after diagnosis or in pre-symptomatic patients identified by screening — may be even more effective.
Through the blood test, they also tested for glycated hemoglobin (HbA1c), which indicates a person's average blood sugar level over the past three months. The HbA1c levels of both groups of patients were comparable, despite the treated group making more insulin.
Thomas attributed this result to the study's design. The researchers had all the participants aim for a target HbA1c value: if their body made more insulin, they would inject less insulin to reach that average blood sugar, while if they made less, they had to inject more insulin to reach that target.
These results didn't surprise the team, because they aligned with observations from previous research conducted on diabetic animals, Thomas said.
Autoimmune reactions involve an enzyme family known as Janus kinases (JAK), whose activity baricitinib inhibits. Previous work from the research group showed that JAK inhibitors disrupt the interaction between immune cells and beta cells, preventing beta cell death. That's why they hypothesized that this class of drugs could potentially slow the progression of type 1 diabetes.
"The trial confirmed what we had suspected," Thomas told Live Science.
The authors noted the trial's limitations, such as the small number of patients included and the short duration of the trial that prevented them from detecting rare side effects. What's more, unlike a drug approved last year to prevent type 1 diabetes progression, which is given during one time period, baricitinib would likely need to be taken continuously to keep working.
The next step involves discussing the trial's results with the Food and Drug Administration, Thomas said.
"While the drug is already approved for other diseases,it is likely that a further trial will be needed before it is approved for type 1 diabetes," she said.

Sneha Khedkar is a biologist-turned-freelance-science-journalist from India. She holds a master's degree in biochemistry and a bachelor's degree in microbiology and biochemistry. After her master's, she worked as a research fellow for four years, studying stem cell biology. Her articles have been published in Scientific American, Knowable Magazine, and Undark, as well as several Indian platforms such as The Hindu and The Wire Science, among others. Besides writing, she enjoys a good cup of tea, reading novels and practicing yoga.
What are mRNA vaccines, and how do they work?
Deadly motor-neuron disease treated in the womb in world 1st