Lab-made universal blood could revolutionize transfusions. Scientists just got one step closer to making it.
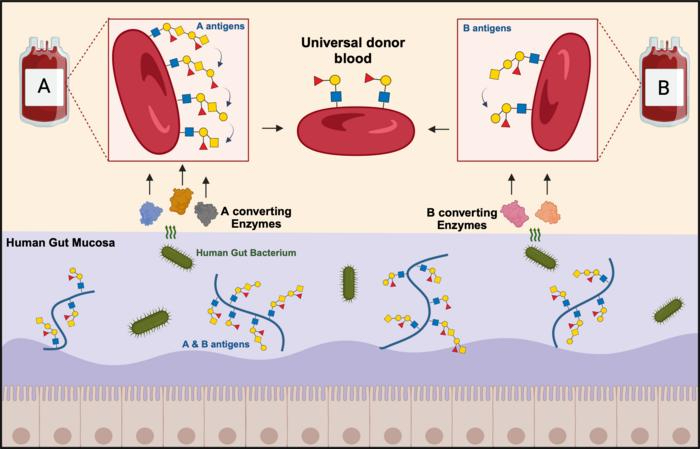
Enzymes produced by gut bacteria can remove long sugar chains in type A and B blood, leading to improved compatibility with type O.
Using gut bacteria, scientists have come one step closer to "universal" donor blood, where any blood type can donate to any other.
While people with type O blood are already universal donors, there isn't always enough of this blood type to go around. So finding a way that people with any type of blood can donate to others could reduce the chances of blood shortages. Still, much more work needs to be done before this method could reach the clinic.
In the new study, published April 29 in the journal Nature Microbiology, researchers identified long strings of sugar molecules that make blood donations from one blood type, such as A, incompatible with recipients who have another type. Then, they used a cocktail of gut bacteria enzymes to strip those long sugar extensions from red blood cells (RBCs).
"Instead of doing the work ourselves and synthesizing artificial enzymes, we've asked the question: What looks like a red [blood] cell surface? The mucus in our gut does. So, we simply borrowed the enzymes from the bacteria that normally metabolize mucus and then applied them to the red [blood] cells," Dr. Martin Olsson, a professor of hematology and transfusion medicine at Lund University in Sweden, told Live Science. "If you think about it, it's quite beautiful."
Related: Why do we have different blood types?
Getting the wrong type of blood transfusion can lead to a fatal immune reaction. That's because the immune system will recognize, and launch an attack on, foreign sugar molecules — or antigens — that protrude from RBCs. The A antigens in type A blood don't mix with the B antigens in type B blood. Type O, the universal donor blood, lacks these antigens, which is why it can be transfused into people with any blood type.
For decades, scientists have been trying to use enzymes to clip off the antigens in type A and B blood. Starting with using enzymes from unroasted coffee beans to convert type B blood to type O in the 1980s, scientists have since found faster and better enzymes that work in both type A and B blood. After stripping these RBCs clean of known antigens, both type A and type B blood look molecularly like type O blood.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
But when this treated blood is mixed with type O blood plasma - the watery part of blood, the latter reacts positively with the former, signaling incompatibility.
"There was no A or B left. How can they be incompatible when they, according to all the books, should be compatible?" Olsson said.
It turned out that the scientists just needed to take a closer look. When they did, they found that type A and B stripped of these well-known antigens still harbored long chains of sugar molecules, called extensions, which also seemed to lead to incompatibility.
In the new study, Olsson and colleagues showed that removing the antigens and the extensions from type A and B blood makes it more compatible with type O blood. The group used a cocktail of enzymes from Akkermansia muciniphila — a type of bacteria in the human gut that breaks down these long sugar chains in the mucus lining the intestine.
When the scientists removed the original antigens in type B blood and tested it in the lab with type O plasma, about 80% of B donor plasma was compatible with type O plasma. This went up to about 91% to 96% once the extension was also removed, which suggested that the extensions may have contributed to the initial incompatibility.
Related: In a 1st, two people receive transfusions of lab-grown blood cells
The results are not as straightforward for type A blood, with only 20% of A donors initially not causing a reaction. That increased to about 50% after the extensions were removed. Type A appears to be more "biochemically complicated" than type B, said Dr. Steven Spitalnik, co-director of the Laboratory of Transfusion Biology at Columbia University, so the scientists will need to revise their cocktail of enzymes to do a cleaner job there.
While much more work will need to be done before this method could be safe enough to be used in real blood transfusions, it's a first step.
"The hunt has been on for the magic enzyme or the magic cocktail of enzymes, and this has gotten really close," Spitalnik, who was not involved in this work, told Live Science."Now one has to prove that this is safe and that the red cells survive in the circulation normally," he added.

Rohini Subrahmanyam is a scientist-turned science writer with a PhD in Biology and postdoctoral experience in Developmental Biology. She mostly likes writing about interesting creatures on our planet, ranging from zombie flies and regenerating worms, to intelligent octopuses and mysterious comb jellies.