Brain signals underlying chronic pain could be 'short-circuited,' study suggests
Scientists uncovered a "biomarker" for chronic pain in the brains of four patients and are using the data to develop a new brain stimulation therapy.
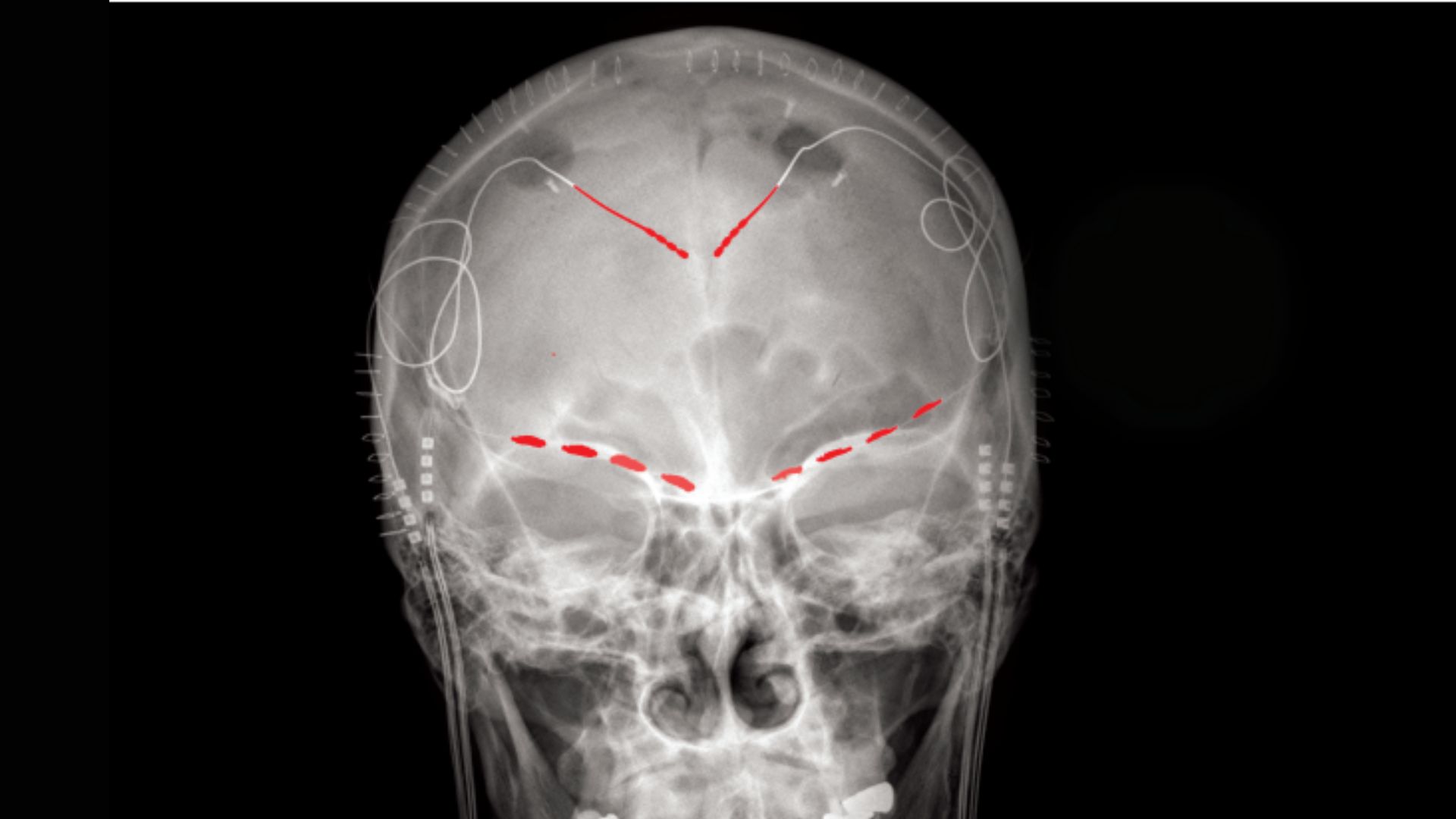
People with chronic pain are often asked to rate their discomfort on a crude 10-point scale. Now, in a first-of-its-kind study, scientists have shown that the fluctuations in pain severity that patients report can be tied to distinct patterns of activity in their brains.
The goal of the research is not to supplant patients' subjective descriptions of their pain with objective brain recordings but rather to find new ways of treating chronic pain with brain stimulation. The idea is that, by pinpointing what chronic pain looks like in a given patient's brain waves, doctors will someday be able to use carefully placed electrodes to short-circuit that patient's pain as it is flaring.
The new study, published Monday (May 22) in the journal Nature Neuroscience, is limited in that it included only four people, but the work is part of an ongoing clinical trial aimed at developing a therapy for these and other patients with hard-to-treat chronic pain. The trial will be followed by a larger one, involving six people, and then an even larger one, involving 20 or 30 people, Dr. Prasad Shirvalkar, a neurologist and interventional pain medicine specialist at the University of California, San Francisco and the study's first author, said at a news conference May 18.
"These patients have tried everything — they've tried medications, injections, and nothing was working," Shirvalkar said of the first four study participants. "The hope is … as we understand this better, we can actually use this information to develop personalized brain stimulation therapies for the most severe forms of pain."
Related: Brain cells gone haywire during sleep may lead to chronic pain, mouse study suggests
The new study stands out from previous research because, rather than measuring people's brain waves in a clinical setting with noninvasive scans, it involved implanting electrodes directly into participants' brains and taking recordings as they went about their daily lives. The implants can both record people's brain waves and deliver electrical stimulation to the organ, which made the implants ideal for the ongoing clinical trial, Shirvalkar said.
The electrodes were implanted in two locations in the front of the brain: the anterior cingulate cortex (ACC), a key region for processing emotions and regulating emotional responses, and the orbitofrontal cortex (OFC), which is also involved in emotional processing, as well as in weighing the consequences of complex behaviors.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The ACC's role in chronic pain has been studied more extensively than the OFC's, the study authors noted in their report. But based on the available research, the team hypothesized that the activity of either brain region might reflect a person's subjective experience of their chronic pain. Therefore, brain waves generated by either region could be used as an objective metric, or a biomarker, of a patient's pain severity, they proposed.
Again, they hoped this biomarker could point towards potential treatments and not be used to supersede patients' subjective experiences, Shirvalkar said.
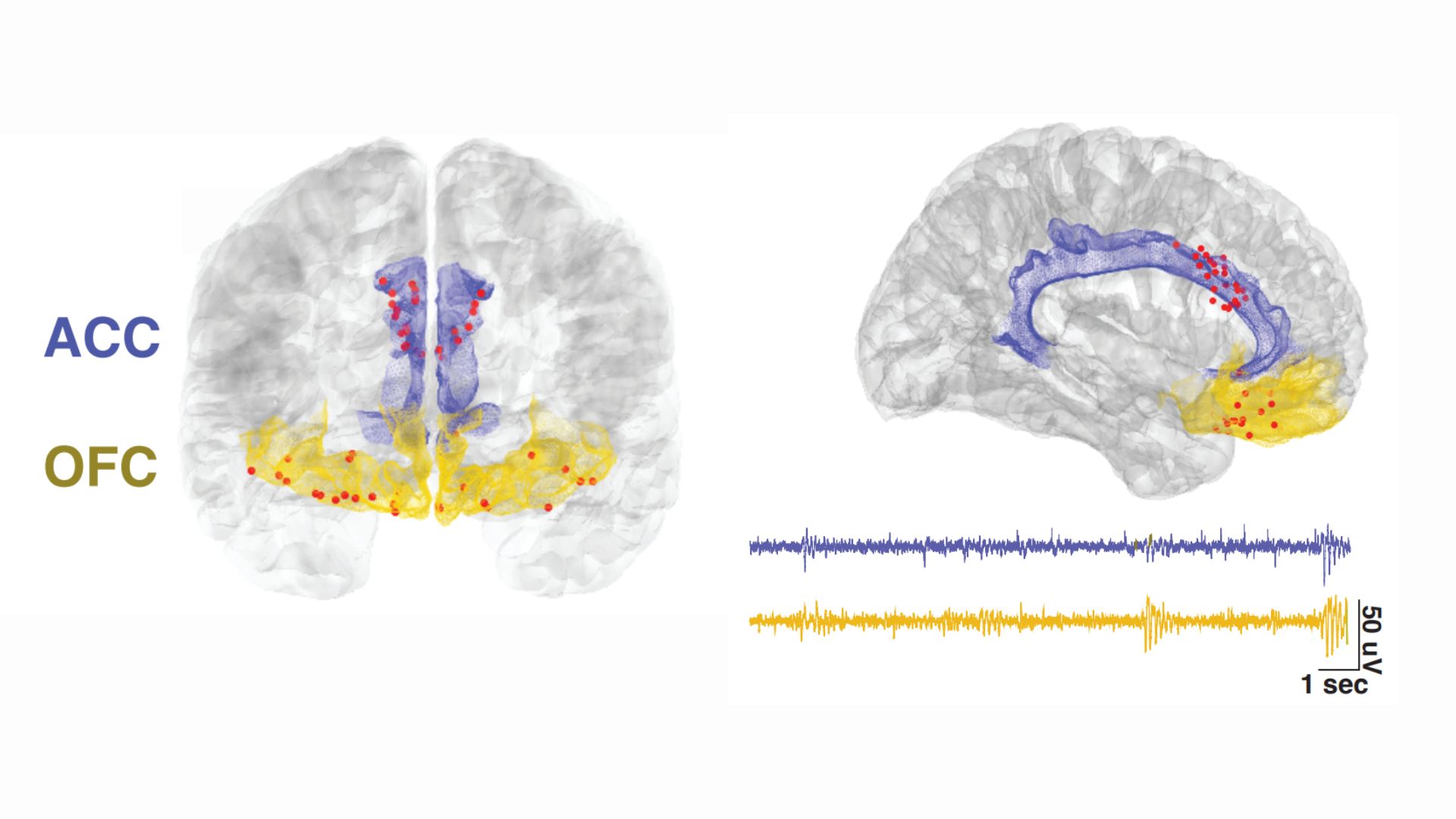
After having electrodes surgically implanted in their brains, the four study participants started completing daily surveys about severity of their pain, as well as the quality of their pain, in terms of its level of unpleasantness and whether it felt like burning or stabbing, for example. They provided between two and eight of these pain reports a day for three to six consecutive months. After logging each pain update, the participant would push a button to cue their implanted electrodes to take a 30-second snapshot of their brain activity.
All this data got fed into a machine learning algorithm, which identified consistent patterns in how each individual's pain and brain activity shifted over time. The resulting personalized computer models could eventually be used to predict the level of pain a participant was experiencing based on their brain signals. And specifically, the activity of the OFC, not the ACC, was useful for making these predictions.
"What we saw is each patient's biomarker was actually like a unique fingerprint," Shirvalkar said.
In addition to the at-home portion of their study, the team ran an experiment in which each participant experienced acute pain caused by heat in the lab. They found that this heat-related pain resulted in patterns of brain activity that were distinct from those tied to chronic pain and, by contrast, were reflected mostly in the ACC.
This underscores the idea that "chronic pain is not just a more-enduring version of acute pain. It's actually fundamentally different in the brain," Shirvalkar said.
He added that as all four study participants had neuropathic chronic pain, or pain caused by nerve damage, rather than nociceptive pain, or pain triggered by an injury to bodily tissues, it's not yet clear if the same brain wave patterns would be seen in nociceptive chronic pain. This could be the subject of future studies, but the current trials are focused on neuropathic pain.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.