At-home brain stimulation could be promising depression treatment, trial hints
A new trial suggests that at-home brain stimulation could potentially be a first-line treatment for depression. However, some experts are skeptical.

Brain stimulation is an evidence-backed treatment for depression — and now, a clinical trial supports the idea that patients could do it themselves at home.
The trial showed that, under remote supervision, 87 patients with depression could successfully use a headset that delivers a weak electric current to a specific part of the brain. This kind of treatment, known as transcranial direct current stimulation (tDCS), would normally be given in a clinic.
After regularly using the headset for almost three months, these patients showed significantly greater improvement in their symptoms compared to a comparison group of 87 patients who followed the same procedure but with headsets that didn't deliver any electric current.
The findings, published Monday (Oct. 21) in the journal Nature Medicine, demonstrate that this approach could be a potential first-line treatment for depression, Dr. Cynthia Fu, study co-author and a professor of affective neuroscience and psychotherapy at King's College London, told Live Science. (Fu and colleagues received funding for the trial from the company that developed the device.)
However, experts not involved with the research told Live Science that issues with the trial's design could limit how well the research applies to people with depression, at large.
Related: 6 distinct forms of depression identified by AI in brain study
Around one-third of patients with depression still fail to see an improvement in their symptoms with first-line treatments, such as antidepressant drugs. Because of this, there's a demand for alternative therapies for the disorder, such as tDCS.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
During a typical tDCS session in a clinic, a flexible cap or band fitted with electrodes is placed on a patients' scalp. A mild electric current is then applied through the electrodes to specific regions of the brain, most commonly the prefrontal cortex. This part of the brain is found just behind the forehead and its function is known to be impaired in depression. The electrical current tDCS delivers to the prefrontal cortex is thought to make it easier for neurons to fire, or to send signals to each other. Typically, patients come in for daily sessions for several weeks.
Previous clinical trials have suggested that tDCS could feasibly be used by patients at home under remote clinical supervision, like a video call. However, these trials have produced inconclusive results about how well at-home tDCS relieves symptoms.
In the new trial, Fu and colleagues developed their own version of an at-home tDCS device. They enrolled 174 patients in the U.S. and the U.K. who were in a depressive episode of "moderate severity." In this case, this meant each patient scored more than 17 on the Hamilton Depression Rating Scale (HDRS), a standardardized scale that clinicians use to measure the severity of a patients' depressive symptoms.
The researchers split the cohort in two. One half — the "active" treatment group — was instructed to use the headset at home in 30-minute sessions, repeated several times a week for 10 weeks. The control group also did this, but their headsets didn't deliver any stimulation. Both groups were guided by a doctor via videoconference.
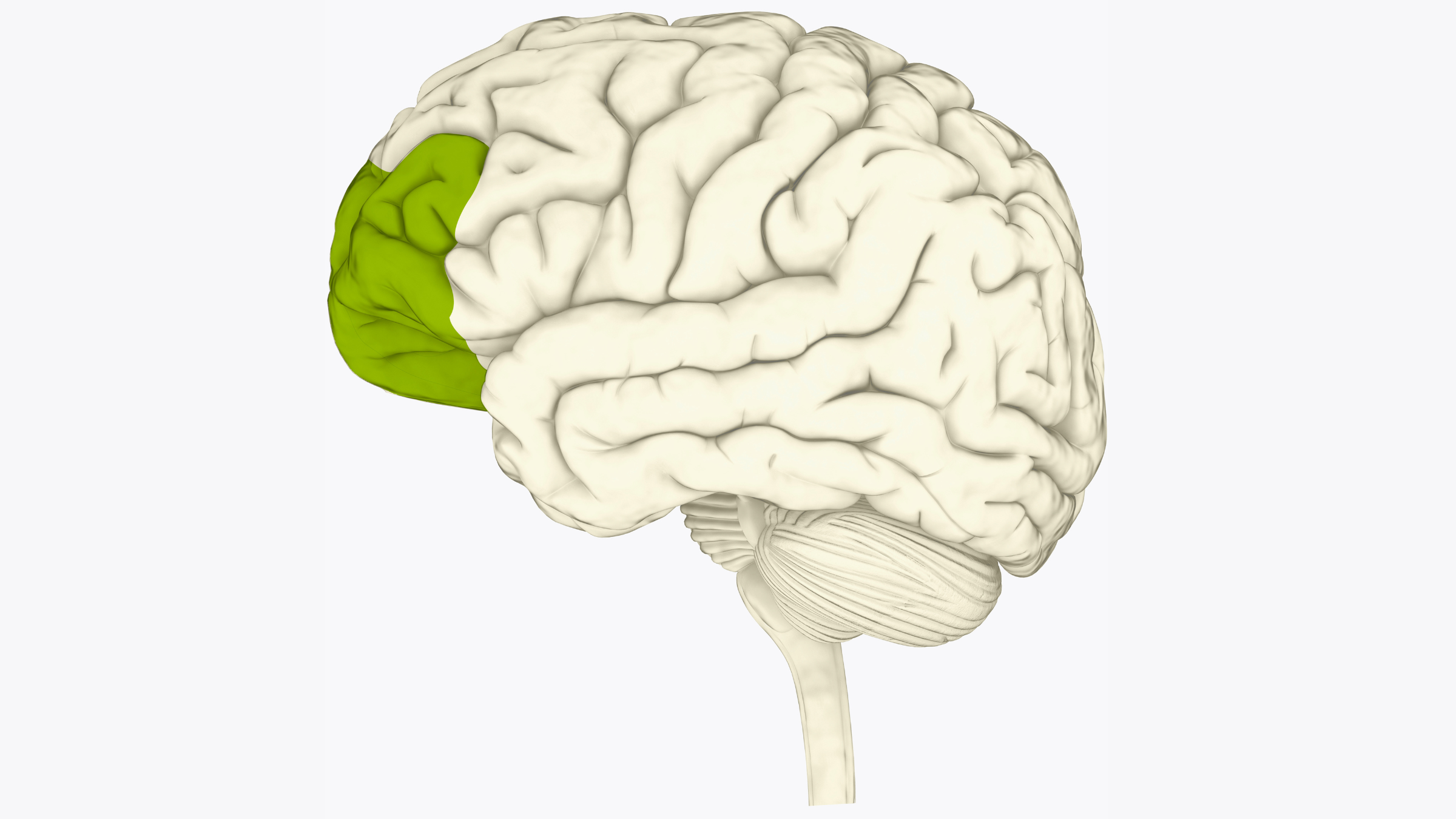
Over the 10 weeks, both groups saw significant improvements in their depressive symptoms. HDRS scores improved by an average of 9.41 for the active treatment group and 7.14 for the control group. A significantly greater percentage of patients in the treatment group achieved clinical remission — about 45% compared to 22% of the control group. Remission means that a patient no longer has depressive symptoms.
Overall, 13 patients in the active treatment group and 12 in the control group discontinued treatment.
Limits to the research
While these results appear encouraging, there were several limitations of the trial.
For example, many patients correctly guessed whether they received tDCS or not, said Jonathan Roiser, a professor of neuroscience and mental health at University College London who was not involved in the research. This was probably because of minor side effects that can occur with tDSC, such as skin redness, Roiser told Live Science in an email. This may have potentially biased the findings because patients who knew they got the real treatment might have an inflated sense of how much their symptoms are improving.
Furthermore, the researchers noted in their paper that the study population mainly included white people, Dr. Sarah Lisanby, director of the Division of Translational Research at the National Institute of Mental Health who was not involved in the research. It's therefore uncertain if the same treatment would work for all demographics, she said.
The study also excluded patients with more severe forms of depression, which may also limit how well the findings apply to people with worse symptoms, she said.
Despite these constraints, the findings of the trial are still valuable, Lisanby said.
"Anything that we as a field can do to improve access to safe and effective mental health care is worth studying," she said.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30. (emily.cooke@futurenet.com)