Scientists make breasts in a dish to study lactation
Scientists are making mammary gland organoids with 3D milk ducts that could shed light on lactation and breast cancer development.
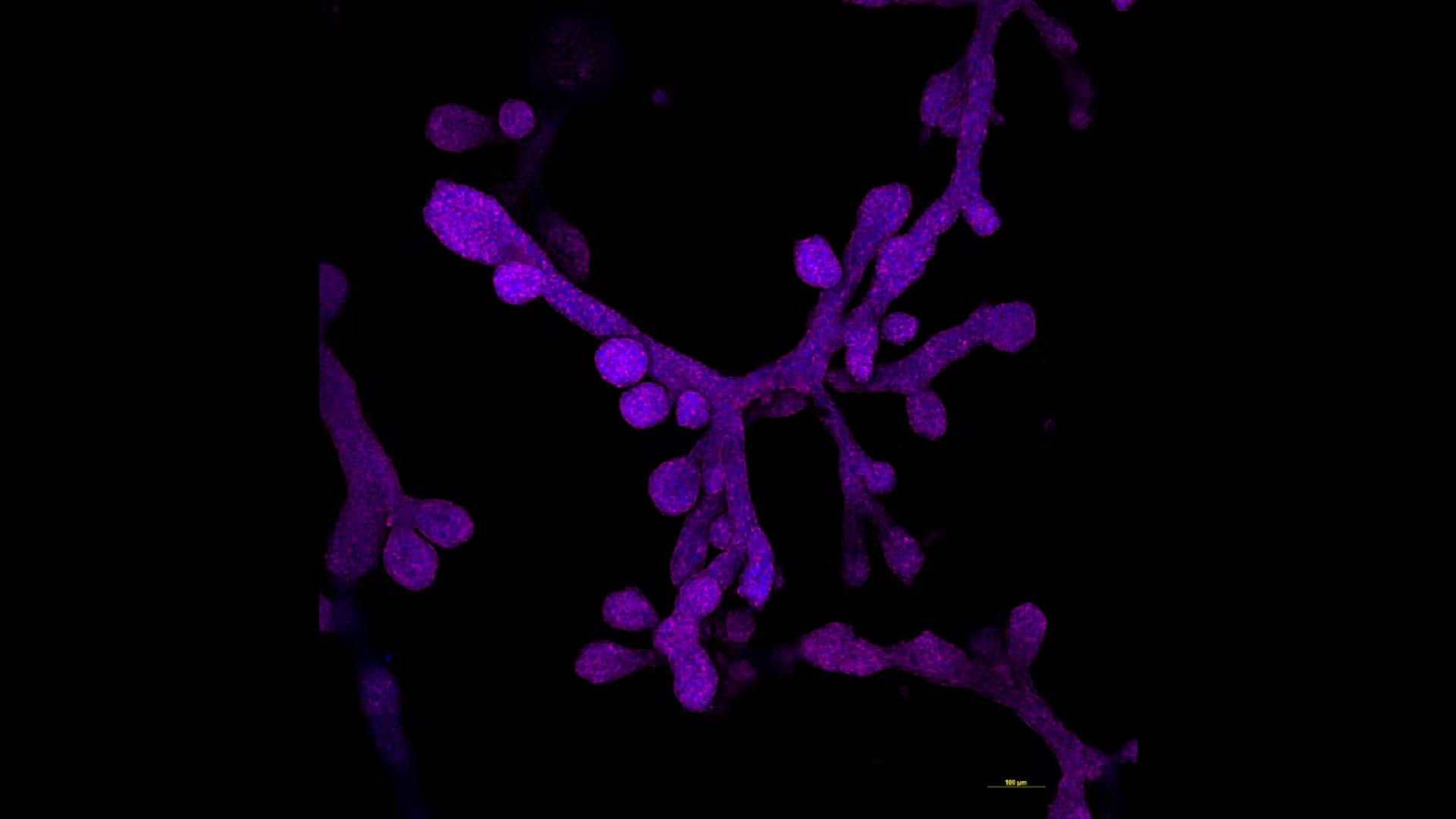
All mammals have mammary glands that produce milk, a feature that has fascinated scientists for many years. Questions such as why mammary glands evolved in the first place, how they have adapted across different species and what unique evolutionary pressures shaped their development remain largely unanswered.
To investigate how various species have evolved unique solutions to biological challenges, my team at the Rauner Lab of Tufts University School of Medicine is recreating mammalian diversity in a dish through miniature versions of mammary glands — organoids. These models can shed light on the fundamental biological processes behind milk production, tissue regeneration and the early stages of breast cancer development.
What are organoids?
Organoids are miniature, 3D structures grown in a cell culture dish that mimic the structure and function of real organs. These models are made by guiding stem cells, which have the unique ability to differentiate into various types of cells, to form specific types of organ cells.
While they are not exact miniature replicas of full-size organs, organoids contain enough cells and tissue architecture to recreate the environment and key functions of the organ they model. For example, a mammary gland organoid or a breast tissue organoid are composed of tiny elongated ducts that terminate in a spherical structure, mimicking the milk ducts and alveoli of the gland's tissue.
Related: Could mini space-grown organs be our 'cancer moonshot'?
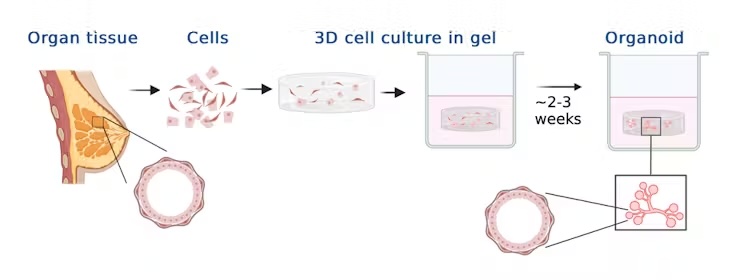
Organoids provide a powerful tool for biomedical research because they offer a 3D representation of an organ's structure and function. Unlike traditional 2D cell cultures, organoids can mimic the complexity of actual tissues, including their architecture and diverse cell types. This enables researchers to study complex biological processes such as tissue development, regeneration and disease progression, in a controlled environment, while reducing reliance on animal models.
Mammalian diversity in a dish
Researchers have traditionally used organoids to model human diseases, test drugs and study developmental biology. However, their potential extends far beyond these applications, particularly in the field of evolutionary biology.
My research focuses on generating mammary gland organoids from a variety of mammalian species. Mammals are incredibly diverse, with each species adapted to a wide range of environments and lifestyles. The mammary gland, essential for nurturing offspring, exhibits significant variation across species.
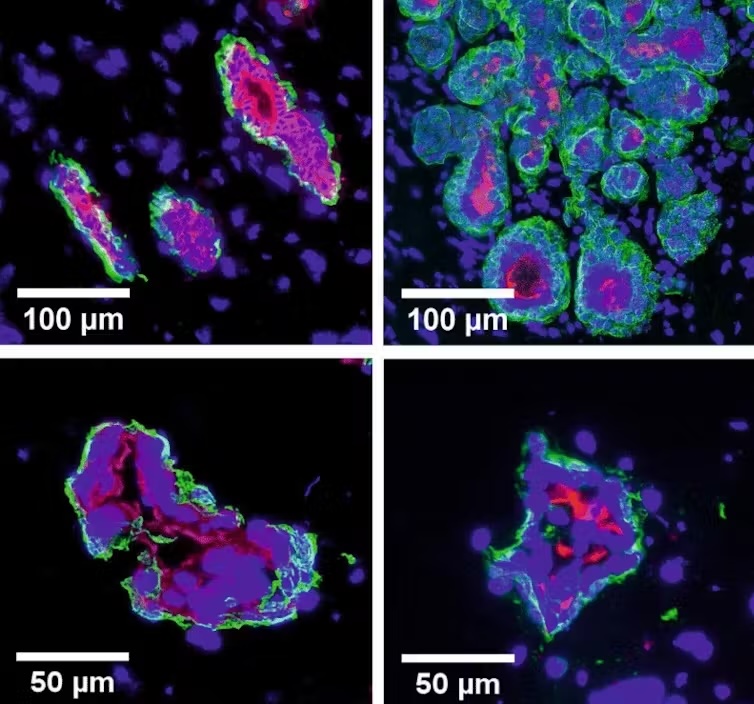
For instance, monotremes such as the platypus and echidna belong to a unique and ancient class of mammals. Monotremes diverged from other mammalian groups approximately 190 million years ago and are distinguished by their reproductive methods: laying eggs instead of giving live birth. Their mammary glands are markedly different from those of eutherian mammals such as cows and humans that have nipples; monotremes instead secrete milk through specialized mammary hairs.
Scientists believe that different environmental pressures and reproductive strategies have driven the evolution of diverse forms of lactation. However, the exact mechanisms and evolutionary pathways are still largely unknown. By comparing organoids from these diverse species, researchers can shed light on how these ancient structures have evolved and adapted over millions of years to meet the reproductive needs of different animals.
Insights beyond the mammary gland
Studying the mammary gland's unique properties can also shed light on other areas of biology and medicine.
For example, the mammary gland is able to regenerate with each cycle of reproduction and lactation. That makes it an excellent model for studying tissue regeneration. With organoids, researchers can observe the process of regeneration in real time and investigate how different species have evolved to maintain this regenerative capacity. Understanding the mechanisms behind regeneration could lead to advancements in regenerative medicine, a field that focuses on repairing or replacing damaged tissues and organs in conditions such as heart disease, diabetes and injuries.
Mammary organoids can also help with breast cancer research. Studying mammary organoids from species that rarely develop breast tumors, such as cows and pigs, could uncover potential protective mechanisms and inform new strategies for breast cancer prevention and treatment in people. Organoids also provide a platform to study the early events of tumor formation and the cellular environment that contributes to cancer development.
Organoids also enable scientists to study the initiation, duration and cessation of lactation in different species. The process of lactation varies widely among mammals, influenced by factors such as hormonal changes and environmental conditions. Some mammals have unique forms of lactation. For example, marsupials such as the Tammar wallaby can produce two types of milk simultaneously to meet the nutritional needs of offspring at different developmental stages, a phenomenon known as asynchronous concurrent lactation. Additionally, the fur seal can maintain lactation despite extended periods without nursing.
Studying different types of lactation through mammary organoids can provide deeper insights into how lactation is regulated, revealing evolutionary adaptations that could clarify the biology of human lactation and improve livestock milk production strategies in agriculture.
The potential of organoid technology
Organoids offer several advantages over traditional animal models. For one, they provide a controlled environment to study complex biological processes and enable scientists to conduct multiple tests simultaneously, increasing research efficiency.
They also reduce the ethical concerns associated with animal research. Organoids can be generated from animals that are not available for live research, such as rare or endangered species.
Moreover, organoids can be genetically modified to investigate specific genes and pathways, providing deeper insights into the molecular mechanisms underlying mammary gland biology.
While organoids are a powerful tool, they are not without limitations. They cannot fully replicate the complexity of living tissues, and findings from organoid studies must be validated in living subjects. Despite these hurdles, advancements in organoid technology continue to push the boundaries of what is possible, offering new opportunities to explore mammalian diversity and evolution.
By recreating the diversity of mammalian tissues in a dish, researchers can gain important insights into how different species have evolved to solve biological challenges, with the potential to benefit human health, agriculture and nutrition science.
This edited article is republished from The Conversation under a Creative Commons license. Read the original article.
Get the world’s most fascinating discoveries delivered straight to your inbox.

Tissue-resident adult stem cells are the source of organ and tissue development, regeneration and maintenance. In many tissues they are also suspected as cancer cells-of-origin, both due to their long life-span as well as their self-renewal and regenerative capacities. As such, adult stem cells are a link that connects the closely related biology of development and tumorigenesis. It is becoming increasingly clear that stemness is a cell state, rather than a final cell identity. Cells shift their state as part of normal development and of oncogenic progression and invasion. The regulatory mechanisms that control shifts in cell state also control development and cancer and appear to involve all levels of regulation: from genetic, epigenetic and epi-transcriptomic to post-transcription, post-translation and classic cell signaling pathways. I am interested in understanding the regulatory mechanisms that control adult stem cells in the contexts of development and cancer, particularly in the earliest stages of tumorigenesis, when the developmental program deviates towards cell transformation.

