2nd tuberculosis outbreak linked to bone grafts in the US
Following two recent outbreaks, health officials have issued new guidance around how to screen donated tissues for tuberculosis.
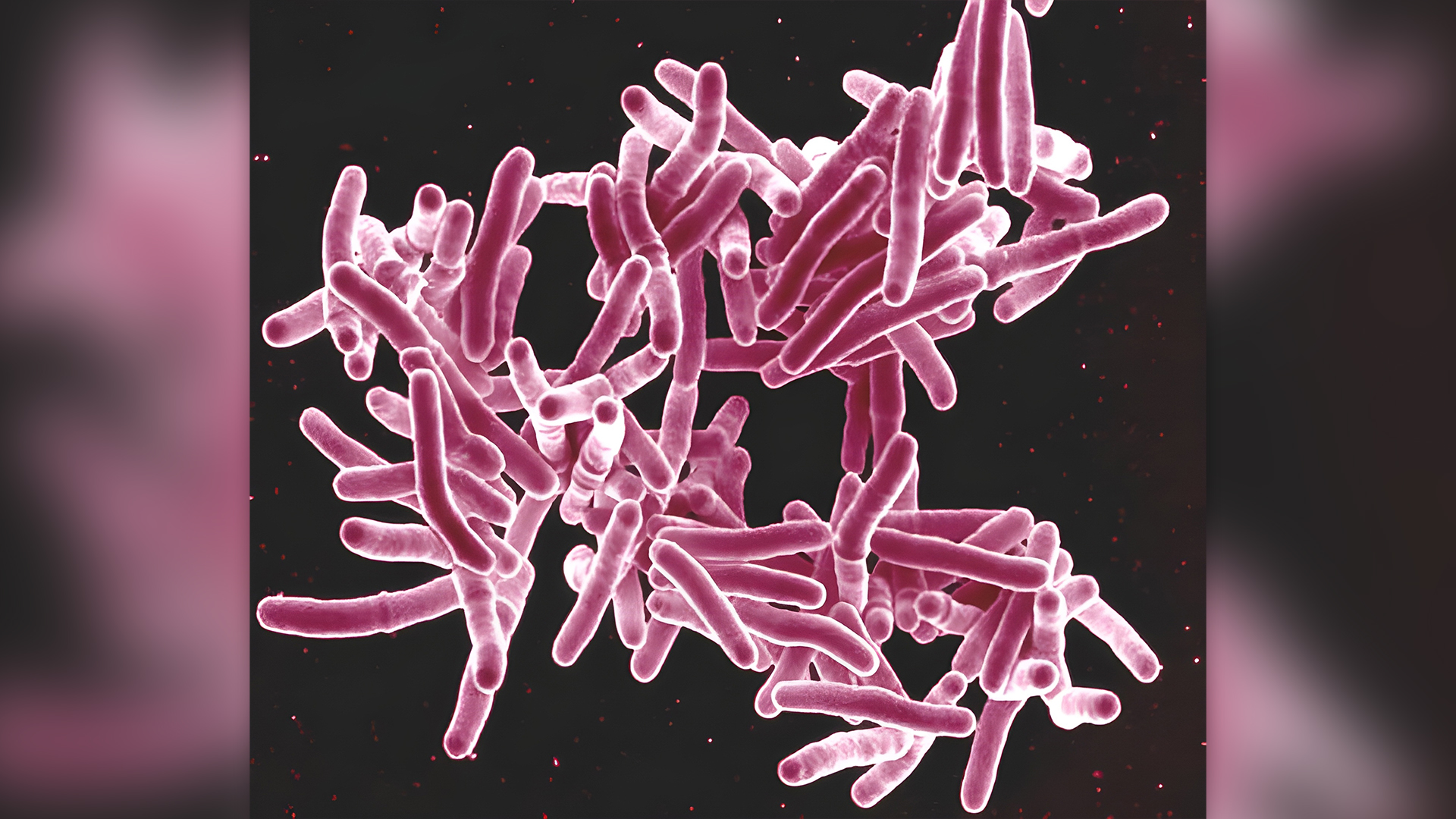
An outbreak of tuberculosis that affected dozens of people last year has been tied to bone grafts, echoing a larger outbreak in 2021.
These two recent outbreaks highlight the need to better screen donated tissues for tuberculosis-causing bacteria before they're used in such medical procedures, scientists wrote in a Morbidity and Mortality Weekly Report (MMWR) published Friday (Jan. 5) by the Centers for Disease Control and Prevention (CDC).
During the 2021 outbreak, 113 people who underwent spinal surgeries were exposed to Mycobacterium tuberculosis, the bacterium behind tuberculosis, via a bone-repair product. The product — called FiberCel and made by Elutia (formerly Aziyo Biologics) — is a putty derived from donated human bone tissue that contains live cells. The FiberCel batch contaminated with M. tuberculosis had come from one deceased donor's tissues.
The 2023 outbreak strongly resembled the previous one: It involved a similar bone-repair product made by Elutia, and the contaminated batch came from one tissue donor, according to the MMWR.
Related: 28 devastating infectious diseases
The CDC learned of the outbreak in July 2023, when two state health agencies reported tuberculosis infections in two patients who'd undergone spinal surgeries. Both patients had been exposed to the bone-repair product, and both subsequently died of tuberculosis. (Tuberculosis is curable with a months-long course of antibiotics; the MMWR didn't note when the two patients started treatment, whether their treatment was interrupted, or other possible reasons as to why their cases were fatal.)
The CDC launched an investigation in collaboration with the Food and Drug Administration (FDA), and the product's manufacturer issued a voluntary recall of all of its "viable bone matrix products," including the contaminated one. At the CDC's and FDA's request, the company also quarantined all of the product from the contaminated batch that had yet to be distributed.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The part of the batch that had already been distributed had been shipped to eight hospitals and five dental offices in seven states. Federal health officials contacted those facilities and learned that, including the two previously reported cases, 36 total patients had undergone procedures in which the product was used.
Five of the 36 people were diagnosed with tuberculosis based on laboratory testing, while additional people showed less-definitive signs of infection. Regardless, the CDC recommended that all exposed patients receive prompt tuberculosis treatment, and no additional people died.
Leading up to the 2023 outbreak, Elutia had screened its products for M. tuberculosis using a test known as nucleic acid amplification, which looks for genetic material from the bacteria. This test suggested that the contaminated batch was negative for the pathogen when, in fact, it wasn't.
"Although extremely useful for diagnosing TB disease, nucleic acid amplification tests are less sensitive than are the slower culture-based tests for identifying M. tuberculosis," the MMWR notes. In the future, these slower tests — which can take up to eight weeks to deliver final results — should be included in safety testing, the report states.
In addition, tissue donors' medical records should be carefully combed for signs of possible tuberculosis infection.
The donors tied to the 2021 and 2023 outbreaks had no documented diagnosis of the disease. However, both developed sepsis, which can be caused by tuberculosis, and the latter had some clinical symptoms that can be triggered by the infection. Tuberculosis can go undiagnosed when a person has "latent" disease, which causes no symptoms, or because the symptoms of "active" tuberculosis overlap with those of many other illnesses and may not be recognized. Diagnostic tests for the disease are also imperfect and may not catch all cases.
"Persons with evidence of sepsis should be determined to be ineligible for tissue donation," the MMWR states. In addition, the FDA recently issued other guidance for the proper protocol for screening donor tissues for M. tuberculosis.
The likelihood of catching tuberculosis from a bone graft is very, very low, but as the tissue transplant industry grows, this safety gap needs to be closed, the MMWR states.
"Because tissue allografts containing live cells are stored frozen and have expiration dates months or even years after manufacture, ample time exists for both culture-based testing and additional scrutiny of donor medical records," the report adds.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.