What's the difference between gram-positive and gram-negative bacteria?
There are two main types of bacteria, and these categories reflect the microbes' biology and their vulnerability to different antibiotics.

Most species of bacteria can be broadly divided into two groups, known as gram-positive and gram-negative. These categories reflect big differences in the microbes' biology, and they also dictate how doctors treat bacterial infections.
But what are the differences between gram-positive and gram-negative bacteria?
The names themselves date back to 1884, when Danish bacteriologist Hans Christian Gram developed a staining procedure to view bacteria under the microscope. First, he applied a dye, called gentian violet, which penetrates both the protective wall and membrane of bacteria, thus staining the material inside. Then, he added the mineral iodine, which formed a complex with the dye that wouldn't break down in water, thus "fixing" the stain in place.
After being washed with alcohol, some bacteria remained blue or purple, while others did not retain the stain. The first group was dubbed "gram-positive," while the latter was designated "gram-negative."
Related: Dangerous 'superbugs' are a growing threat, and antibiotics can't stop their rise. What can?
Gram's stain experiment pointed toward some sort of difference in the structures of various bacterial cells. However, it was not until the early 1950s that scientists started to understand the differences in the chemical composition of bacterial cell walls that makes a difference in the staining.
Differences between gram-positive and gram-negative bacteria
All bacteria have a cell wall composed of mesh-like strands of a big molecule called peptidoglycan, which surrounds the cell membrane. A cell wall provides the bacterial cell sturdiness and helps maintain its shape and internal pressure. However, there are some key differences between the two classes of bacteria.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
First, gram-negative bacteria have a thin cell wall that is about 1.5 to 10 nanometers across, whereas gram-positive bacteria have a thick cell wall measuring about 20 to 80 nanometers.
Second, the cell walls of gram-negative bacteria are surrounded by an outer membrane that has different properties than the inner membrane encased by the wall. This outer membrane is involved in allowing nutrients into the cell and adhering to other, nearby cells, a function that plays a role in infections. Gram-positive bacteria, on the other hand, lack such an outer membrane.
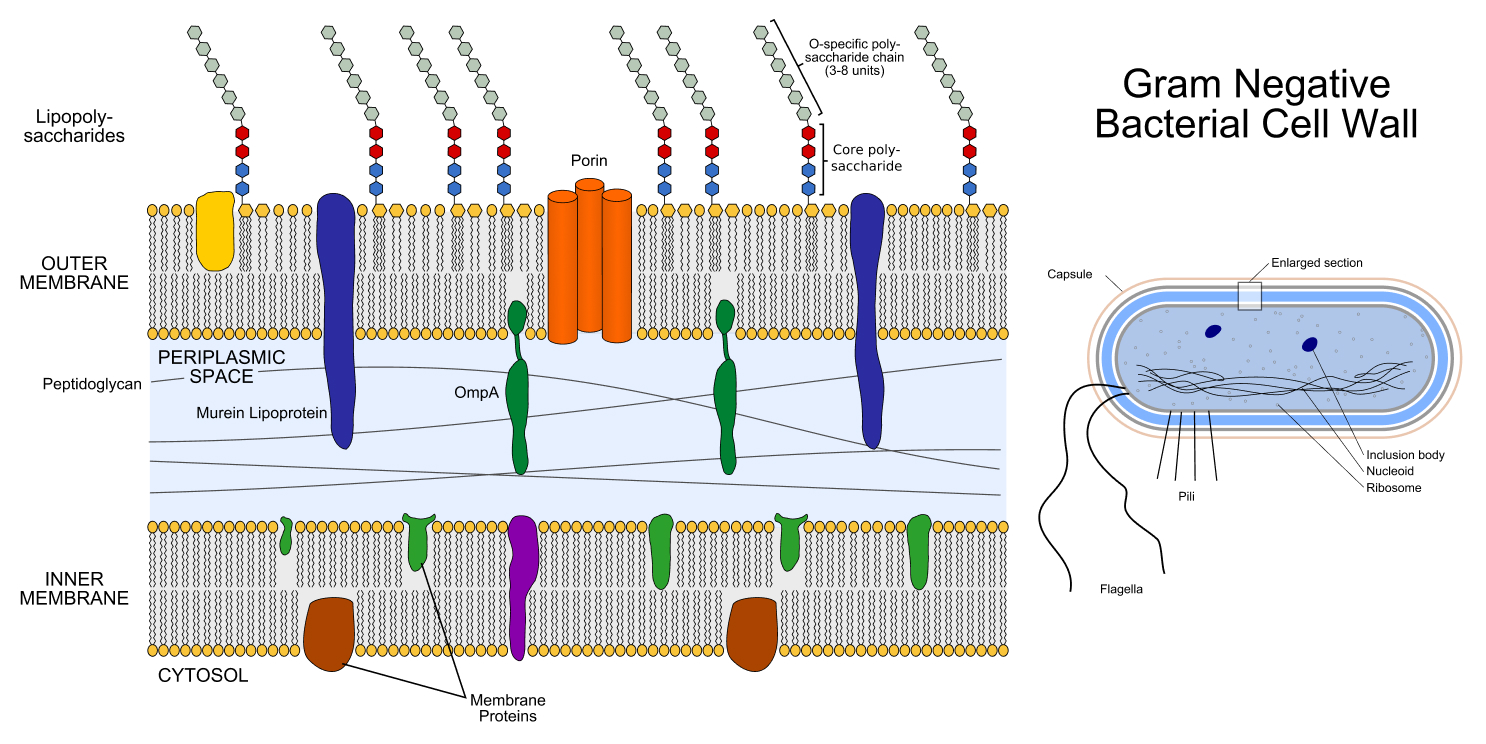
Both classes of bacteria share strategies that they use to resist antibiotics, Mark Blaskovich, a professorial research fellow and group leader at the University of Queensland in Australia, told Live Science in an email. For example, structures called "efflux pumps" in the cell membrane allow bacterial cells to pump out antibiotics that get inside the cell.
Bacterial cells can also make enzymes that chemically inactivate antibiotics; these include beta-lactamase enzymes, which inactivate the class of antibiotics that includes penicillin. Bacterial cells can also alter the parts of their biology targeted by antibiotics, such as proteins or fats; this is equivalent to changing the lock so the key doesn't fit anymore, Blaskovich said.
Bacteria can also pick up antibiotic resistance from neighboring bacterial cells, even if they belong to a totally different species. That's because the microbes can swap small pieces of DNA that carry antibiotic-resistance genes from one bacterial cell to another. These genes can be traded through direct physical contact between the cells in a process called conjugation.
Related: Superbugs are on the rise. How can we prevent antibiotics from becoming obsolete?
All that said, when it comes to antibiotic resistance, gram-negative bacteria have an edge thanks to their double membranes.
The outer membrane of gram-negative bacteria physically blocks some large, water-hating (hydrophobic) antibiotic molecules, such as vancomycin and rifampicin. Because these treatments are blocked from entering the cell, this makes the microbes naturally resistant to the drugs, David Livermore, a professor of medical microbiology at the University of East Anglia in the U.K., told Live Science in an email.
Small, water-loving (hydrophilic) antibiotics can cross the outer membrane, but the membrane still slows down their entry. If a given bacterium has the ability to destroy or pump out the incoming antibiotic, "this is made much more efficient by restricting their rate of entry," Livermore said.
Read more:
—How fast can antibiotic resistance evolve?
—10 of the deadliest superbugs that scientists are worried about
He compared the gram-negative bacterial cell to a castle with small gates in the wall. Because the enemies — antibiotic drugs — are coming in slowly, the defenders — bacteria's defense mechanisms — have an easier time dealing with them. If the battle were on an open field, the enemies would rush in all at once and the defenders would be quickly overwhelmed.
Because gram-negative bacteria are so good at fighting off antibiotics, they pose a particularly big threat to human health, according to the World Health Organization.
The extra outer membrane of gram-negative bacteria generally gives them an advantage over gram-positive bacteria when it comes to beating back antibiotics. However, certain existing drugs, such as polymyxins, and some new experimental drugs target this outer membrane by damaging it or by preventing its manufacture, Livermore said. Currently, these drugs are used as a last-resort treatment for gram-negative bacterial infections that are resistant to multiple antibiotics.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Clarissa Brincat is a freelance writer specializing in health and medical research. After completing an MSc in chemistry, she realized she would rather write about science than do it. She learned how to edit scientific papers in a stint as a chemistry copyeditor, before moving on to a medical writer role at a healthcare company. Writing for doctors and experts has its rewards, but Clarissa wanted to communicate with a wider audience, which naturally led her to freelance health and science writing. Her work has also appeared in Medscape, HealthCentral and Medical News Today.
Flu: Facts about seasonal influenza and bird flu
What is hantavirus? The rare but deadly respiratory illness spread by rodents










