
Humans have been burning coal for thousands of years; since the Industrial Revolution, coal has become a major source of both electricity and global warming. But where does coal come from? By studying how coal forms, scientists can learn both about the deep past and about what to expect when different coals burn.
Coal forms when swamp plants are buried, compacted and heated to become sedimentary rock in a process called coalification. "Very basically, coal is fossilized plants," James Hower, a petrologist at the University of Kentucky, told Live Science. The creation of these plant fossils involves, "a lot of accidents of geology," he said.
Coal formation starts with living plants. "When the tree is still alive, it can be damaged by burning or it can be invaded by insects," Hower said. "All these things will show up in the coal record." Traces of pollen, leaves, roots and even bug poop in coal, Hower said, can be used to reconstruct ancient ecosystems. Fire damage, for instance, gives clues into ancient climates.
Next, plants die. "If the coal is preserved at all, that's telling you something about the overall environment," Hower said. Plants on mountain slopes or in deserts are unlikely to become coal because these environments aren't conducive to the formation of peat.
"Of all coals that we see out there, a very, very high percentage came from swamps," Hower said.
Related: Why is there so much oil in the Arctic?
That's because when plants die in wetlands, they are covered by water and shielded from oxygen. As a result, they do not decay as quickly as they would on dry ground. Instead, plants build up into layers of peat on the soggy bottom of the swamp. That peat, which is sometimes a precursor to coal, has its own long history: it is home to insects, fungi, bacteria and even burrowing tree roots, all of which help break down plants in a process called peatification. "Any one layer we see in a coal could be a product of tens or hundreds or thousands of years," Hower said.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Minerals that seep into peat from the water or that form through chemical reactions are also captured in coal. Fire clay coal in eastern Kentucky, Hower said, contains rare earth elements from a volcanic eruption millions of years ago; the U.S. Department of Energy is now funding technologies to extract these elements from coal waste for use in solar panels, windmills and batteries.
But the minerals in coal also cause problems. Peat exposed to seawater, for example, often contains more sulfur. Burning coal with sulfur comes with an extra human cost; while mining coal and breathing coal smoke are both generally dangerous, high-sulfur coals may be more likely to spontaneously combust in mines and they also may be linked to heart disease.
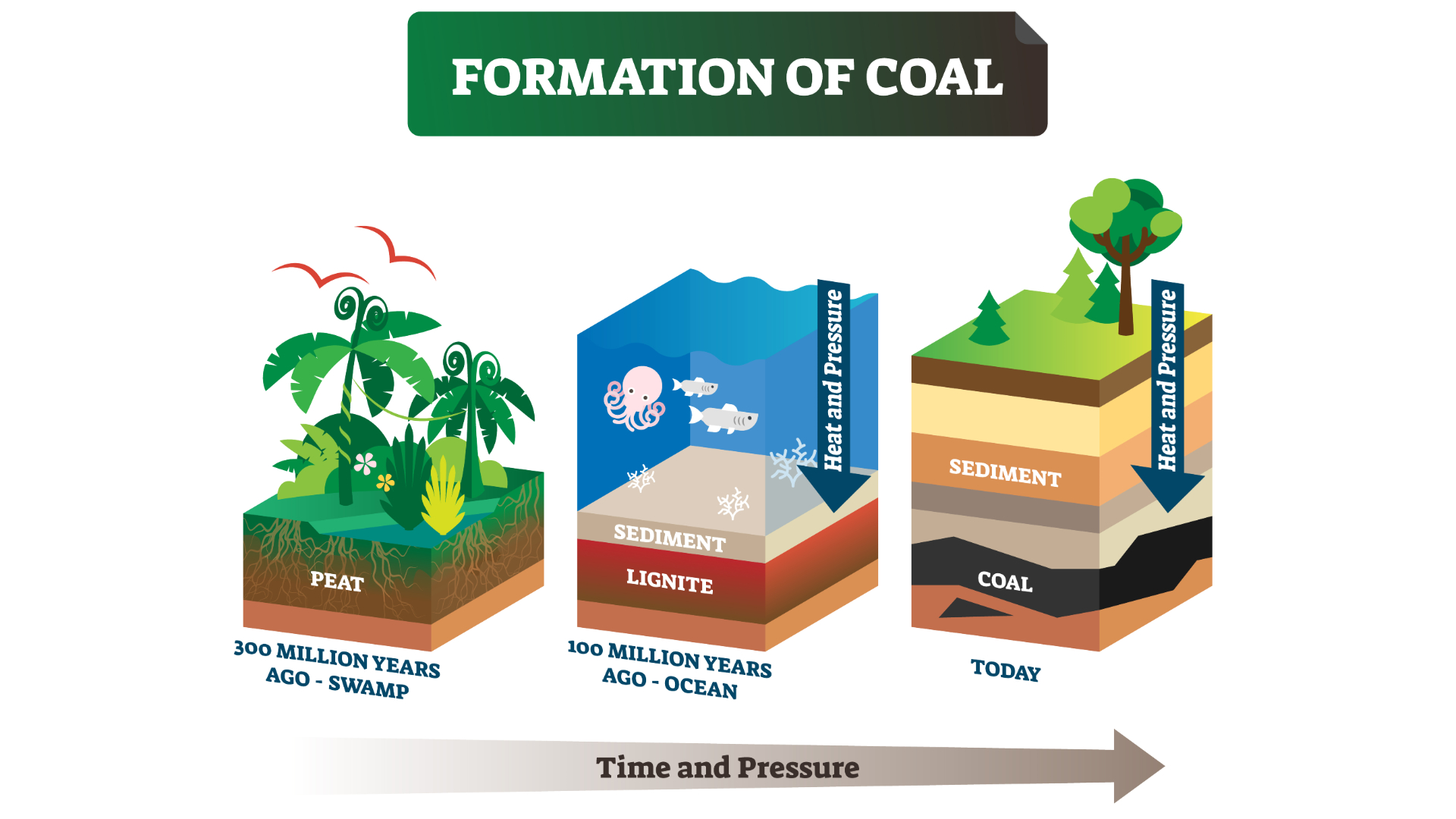
Not all peat transforms into coal; some erodes or dries out. To begin the process of coalification, the peat must be covered by something inorganic, such as silt from a wide river delta. "The river just going back and forth over millions of years, that ends up being your depositional system," Hower said, referring to layers of built up sediment.
Over geologic time, peat is buried even further. Mountains erode and fill up river valleys; forests grow on top. Over millions of years, new mountains rise. During these millennia, the peat breaks down and is gradually transformed to coal thanks to two elements: pressure and heat. Most coals are between 60 million and 300 million years old.
Pressure makes peat more compact. Heat reorganizes the recognizable molecules in plants — like carbohydrates or cellulose — and releases oxygen and hydrogen, leaving carbon and other elements behind.
Coals that are buried very deep experience higher temperatures because they are closer to Earth's core. But geothermal heat can also come to the surface of the Earth through volcanoes, hot springs and geysers. The amount of pressure and heat generally determines the rank of the coal: a measure of how far the coal has progressed in its journey from soggy peat to solid rock.
Lignite is the lowest rank of coal; lignites and sub-bituminous coals still contain recognizable plant parts. Bituminous and subbituminous coals have been compacted and heated until they are hard. Anthracite coal, the rarest and highest rank, is smooth and shiny; it has been heated until fluid in a process called metamorphism. To reach the anthracite rank, Hower said, it is enough to reach a high temperature briefly — even one hour will do the trick.
Anthracites burn without producing soot; they were used historically by coal-powered ships trying to avoid detection in wartime. Lignites and bituminous coals are mostly used for power generation. Lignite and sub-bituminous coals release slightly more carbon dioxide than bituminous coals when they burn.
Those differences are small, however, when coal is compared with other electricity sources that have a lower impact on global heating. In general, coal produces twice as much carbon dioxide per kilowatt hour as natural gas and 90 times as much as wind power, according to the U.S. Department of Energy.
"Emissions from coal and from the industrial processes involved with coal obviously have not been good for the climate," Hower said. "That's the reality we're living in."
Meg Duff is a freelance science journalist and audio producer based in Brooklyn. She holds an M.F.A from New York University's Arthur L. Carter Journalism Institute. Her stories have also appeared in Slate Magazine, Scientific American, MIT Technology Review, and elsewhere.











