Lab-grown mini ‘brains’ of humans and apes reveal why one got so much bigger
Using lab-grown mini-brains, scientists have figured out why humans have bigger brains than those of apes.
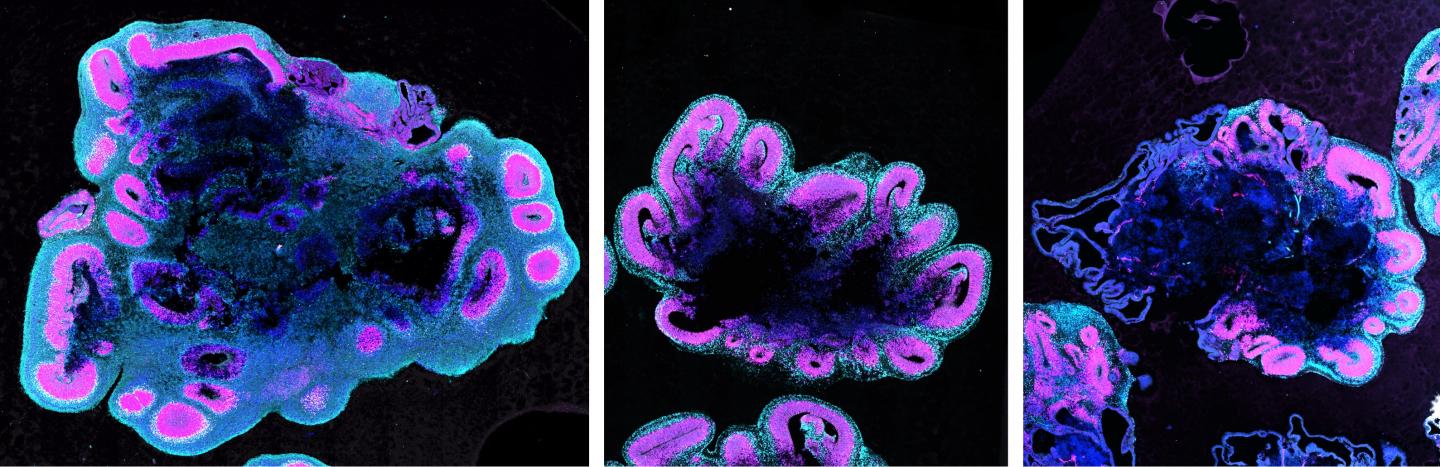
Using lab-grown mini-brains, scientists have figured out why humans have bigger brains than those of apes.
About 5 million to 8 million years ago, humans and apes diverged from a common ancestor. Some time after that, humans started evolving to have larger brains; now human brains are about three times bigger than the brains of chimpanzees, our closest living relatives.
If you ask "what's special about our brains," compared with other apes, the most obvious answer is size, said lead author Silvia Benito-Kwiecinski, a postdoctoral researcher at the MRC Laboratory of Molecular Biology in the United Kingdom. "There's been a strong selection of larger brains and so it would seem that our larger brains have something to do with our unique cognitive abilities."
Related: 8 human-like behaviors of primates
Between 2.6 million and 11,700 years ago, human brains had a major growth spurt, doubling in size, Live Science previously reported. Due to a lack of fossil records dating back to the time of human brain expansion, scientists can't easily tease apart what prompted humans to grow larger brains; but with modern-day tools, we can now see how our brains grow differently than ape brains.
Because human and ape brains rapidly increase in surface area early in development, scientists previously hypothesized that differences might arise very soon after conception, before cells have matured into brain cells, Benito-Kwiecinski told Live Science. But because early human and ape fetal brain tissue is not readily accessible for research, previous studies have mainly focused on later developmental stages when neurons already make up the landscape of the brain.
But the advent of organoid technology, which are models of organs grown in the lab, now makes it possible to look at these earlier stages. Scientists create these brain organoids from stem cells, or cells that can morph into any type of cell in the body, and reprogram those cells to grow into brainlike structures.
Get the world’s most fascinating discoveries delivered straight to your inbox.
While these are not actual brains, they are still impressive mimics; previously, scientists have created brain organoids that could grow their own blood vessels or produce their own brain waves, Live Science previously reported.
In the new study, Silvia Benito-Kwiecinski grew "minibrains" of chimpanzees, gorillas and humans in the lab (this is the first time a gorilla brain organoid has ever been made). They started with 3D balls of cells called embryoid bodies that mimic the early stages of brain development — about a month post-conception — before stem cells mature into brain cells. They then put these cells in gel matrices and allowed them to develop "budding structures" or neural progenitor cells, which are stem cells that will eventually turn into brain cells.
"The reason these progenitor cells are interesting is because, ultimately, the number of neurons generated depend[s] on the number of progenitor cells that are made," Benito-Kwiecinski said. In other words, the more times progenitors divide, the more neurons that will eventually form. These progenitor cells are cylindrically shaped, but as they mature, they start to elongate and become more spindle-like.
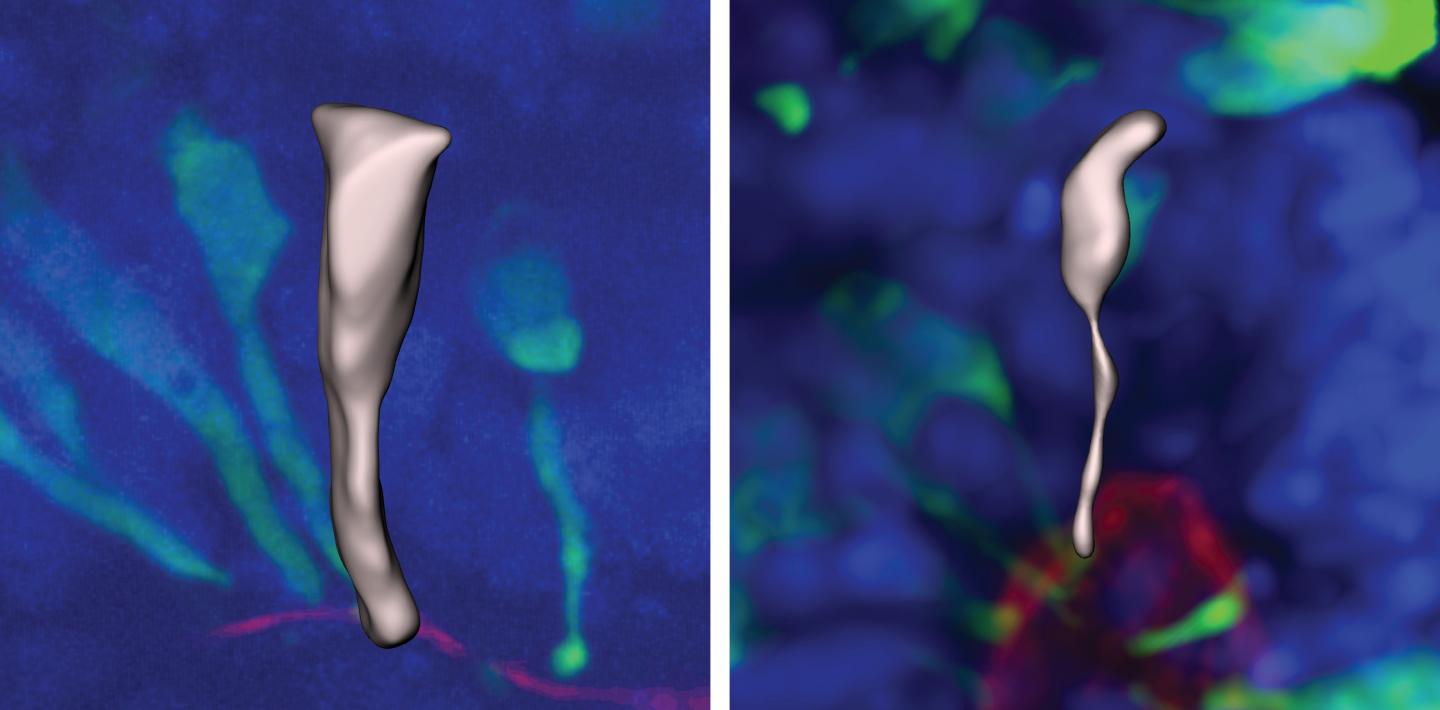
These elongated cells are much slower at dividing than their cylindrical predecessors. Eventually, the spindle-like cells become fully developed neurons.
The researchers found that in human brains, neural progenitor cells take a couple of days longer to mature into these slower-dividing elongated cells than they do in chimpanzee and gorilla brains.
"It appears as if humans are delayed in the transition," to the spindle-like shape, Benito-Kwiecinski said. In that extra time before the transition, human progenitor cells divide more than their ape counterparts, creating more cells that will mature into brain cells, and therefore larger brains.
To understand why, the researchers looked at genes that were turned on and off during this early stage of brain development in the different organoids. They found that the gene ZEB2 was turned on sooner in gorilla brain organoids than in the human organoids. ZEB2 "seems to be the regulator of this cell shape change," Benito-Kwiecinski said.
Sure enough, when the researchers delayed the activation of ZEB2 in gorilla progenitor cells, the transition into the elongated cells took longer, making the cells in the gorilla organoids grow more similar to the cells in human organoids. When they turned on the ZEB2 sooner in human organoids, the opposite happened: The cells in human organoids started growing more like the cells in ape organoids, meaning they transitioned quicker into elongated cells.
It's not clear how soon after humans' split from apes, expression of this gene started to change; and it's also unknown what other genes are involved. Benito-Kwiecinski and her team now hope to understand what regulates the expression of ZEB2, and thus why this gene is expressed later in humans than in apes.
The findings were published Wednesday (March 24) in the journal Cell.
Originally published on Live Science.

Yasemin is a staff writer at Live Science, covering health, neuroscience and biology. Her work has appeared in Scientific American, Science and the San Jose Mercury News. She has a bachelor's degree in biomedical engineering from the University of Connecticut and a graduate certificate in science communication from the University of California, Santa Cruz.
