Scientists finally solve the mystery behind a 100-year-old chemistry experiment
Scientists may finally understand the mysterious transition behind a century-old chemistry experiment. The details of this transformation, in which adding electrons to a bright blue ammonia solution morphs it into a lustrous, metallic bronze, have long eluded scientists.
The new study reveals the subtle details of this change, and shows that this transformation is gradual, rather than sudden. "What we've done successfully is that we've pretty much understood how these solutions behave at a wide range of concentrations using a microjet technique," said study co-author Ryan McMullen, a doctoral student in chemistry at the University of Southern California. This technique, which involves shooting hair-thin streams of the solution through a vacuum, has not been used on the lustrous liquid before.
And the discovery could open up new types of reactions in organic chemistry in the future, McMullen told Live Science.
Related: 8 chemical elements you never heard of
What is a metal?
Metals are a diverse group. Some, like lithium, are light enough to float, while others, like lead or osmium are extremely dense. Some require incredibly high temperatures to melt, while others melt easily (Mercury, for example, melts at minus 38.3 degrees Celsius, or minus 37.9 degrees Fahrenheit). Ultimately, what metals have in common is their ability to conduct electricity at absolute zero, the point at which molecular movement from heat essentially halts.
But how do some nonmetals transform into metals? In a new study, researchers answered that question by adding metals to liquid ammonia.
First, the researchers condensed ammonia, which is a gas at room temperature, into a liquid by cooling it to negative 27.4 F (minus 33 C). They then added either sodium, lithium or potassium, which are all alkali metals. (Rather famously, these metals react explosively when submerged in water.) The experiments were done in collaboration with scientists from the Czech Academy of Sciences and the Fritz-Haber Institute of the Max Planck Society in Berlin, as well as researchers in Japan and France.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Related: The top 10 greatest explosions ever
The result was an expected reaction: The liquid ammonia pulled electrons from the metal. Those electrons then became trapped between the ammonia molecules, creating the so-called solvated electrons the researchers hoped to study. At low concentrations, the result was a blue, non-metallic liquid. As the solvated, or trapped, electrons piled up, though, the solution transitioned to shiny bronze.
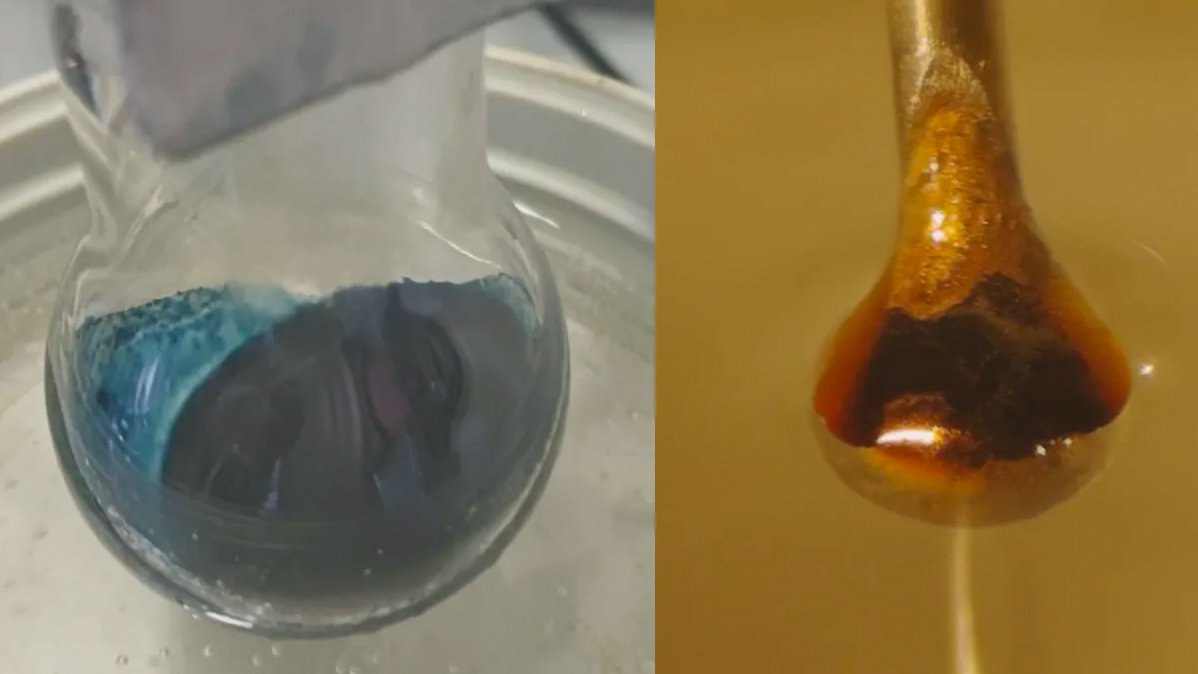
The next challenge was to investigate how the solvated electrons behaved at different concentrations. This involved shooting a microjet of the solution — about the width of a human hair — through a beam of synchrotron X-rays, which are high-energy X-ray beams. The X-rays excited the solvated electrons, causing them to hop out of their liquid cage of ammonia molecules. The researchers could then measure how much energy it took to release the solvated electrons.
The researchers found that the greater the concentration of solvated electrons, the more the pattern of energy release matched what is seen in a metal. Here's what that means: If you graph the amount of energy required to free electrons from their liquid ammonia cage, metals typically have what's called a "Fermi edge," a very abrupt transition, McMullen said. At lower concentrations of solvated electrons, this energy-release graph looks more like a rounded hill. Only at higher electron concentrations did this Fermi edge emerge. The edge reflects how much energy electrons have at a given temperature, McMullen added.
"When you increase the concentration to the metallic range then you see, this wonderful pattern emerges that is very, very characteristic of a metal," McMullen said.
The results were interesting because they showed that the metal-like liquid created by combining alkali metals and ammonia actually is a metal on a fundamental physical level, he said.
"It is a genuine metal, it's not something that just looks like one," McMullen said.
Lower-concentration solvated electrons are used in a type of reaction called a Birch reaction, which adds electrons to molecular structures called aromatic rings. This kind of reaction was used in the manufacture of the first oral contraceptive pills in the 1950s, McMullen said. By understanding how solvated electrons work at high concentrations, researchers can potentially find new kinds of chemical reactions, he said. For example, they might excite the solvated electrons with beams of light to get them to behave in new ways.
"If you tickle the electrons a bit so that they're more energetically excited, you can start looking at some crazy reactions that would never otherwise happen," McMullen said.
The researchers reported their findings June 5 in the journal Science.
Originally published on Live Science.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.










