Cerebral organoids: What are lab-grown 'minibrains'?
Brain organoids, or minibrains, contain human tissues and have potential uses in basic research, drug development and computer science.

In the past decade, lab-grown blobs of human brain tissue began making news headlines, as they ushered in a new era of scientific discovery and raised a slew of ethical questions.
These blobs — scientifically known as brain organoids, but often called "minibrains" in the news — serve as miniature, simplified models of full-size human brains. These organoids can potentially be useful in basic research, drug development and even computer science.
However, as scientists make these models more sophisticated, there's a question as to whether they could ever become too similar to human brains and thus gain consciousness, in some form or another.
How are minibrains made?
Scientists typically grow brain organoids from stem cells, a type of immature cell that can give rise to any cell type, whether blood, skin, bowel or brain.
The stem cells used to grow organoids can either come from adult human cells, or more rarely, human embryonic tissue, according to a 2021 review in the Journal of Biomedical Science. In the former case, scientists collect adult cells and then expose them to chemicals in order to revert them into a stem cell-like state. The resulting stem cells are called "induced pluripotent stem cells" (iPSC), which can be made to grow into any kind of tissue.
To give rise to a minibrain, scientists embed these stem cells in a protein-rich matrix, a substance that supports the cells as they divide and form a 3D shape. Alternatively, the cells may be grown atop a physical, 3D scaffold, according to a 2020 review in the journal Frontiers in Cell and Developmental Biology.
To coax the stem cells to form different tissues, scientists introduce specific molecules and growth factors — substances that spur cell growth and replication — into the cell culture system at precise points in their development. In addition, scientists often place the stem cells in spinning bioreactors as they grow into minibrains. These devices keep the growing organoids suspended, rather than smooshed against a flat surface; this helps the organoids absorb nutrients and oxygen from the well-stirred solution surrounding them.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Brain organoids grow more complex as they develop, similar to how human embryos grow more and more complex in the womb. Over time, the organoids come to contain multiple kinds of cells found in full-size human brains; mimic specific functions of human brain tissue; and show similar spatial organization to isolated regions of the brain, though both their structure and function are simpler than that of a real human brain, according to the Journal of Biomedical Science review.
Although most brain organoids are grown from stem cells, in 2023, a study published in the journal Cell demonstrated that it is also possible to grow them directly from human fetal tissue. Unlike stem-cell-derived organoids, these minibrains don't require scaffolding proteins to support their growth and may be able to more accurately capture brain tissues at specific stages of development, the study authors said.
Why are scientists growing minibrains?
Minibrains can be used in a variety of applications. For example, scientists are using the blobs of tissue to study early human development.
To this end, scientists have grown brain organoids with a set of eye-like structures called "optic cups;" in human embryos in the womb, the optic cup eventually gives rise to the light-sensitive retina at the back of the eye. Another group grew organoids that generate brain waves similar to those seen in preterm babies, and another used minibrains to help explain why a common drug can cause birth defects and developmental disorders if taken during pregnancy. Models like these allow researchers to glimpse the brain as it appears in early pregnancy, a feat that would be both difficult and unethical in humans.
Minibrains can also be used to model conditions that affect adults, including infectious diseases that affect the brain, brain tumors and neurodegenerative disorders like Alzheimer's and Parkinson's disease, according to the Frontiers in Cell and Developmental Biology review. In addition, some groups are developing minibrains for drug screening, to see if a given medication could be toxic to human patients' brains, according to a 2021 review in the journal Frontiers in Genetics.
Such models could complement or eventually replace research conducted with cells in lab dishes and in animals; even studies in primates, whose brains closely resemble humans', can't reliably capture exactly what happens in human disease. For now, though, experts agree that brain organoids are not advanced enough to partially or fully replace established cell and animal models of disease. But someday, scientists hope these models will lead to the development of new drugs and reduce the need for animal research; some researchers are even testing whether it could be feasible to repair the brain by "plugging" injuries with lab-grown human minibrains.
Related: FDA no longer requires animal testing for new drugs. Is that safe?
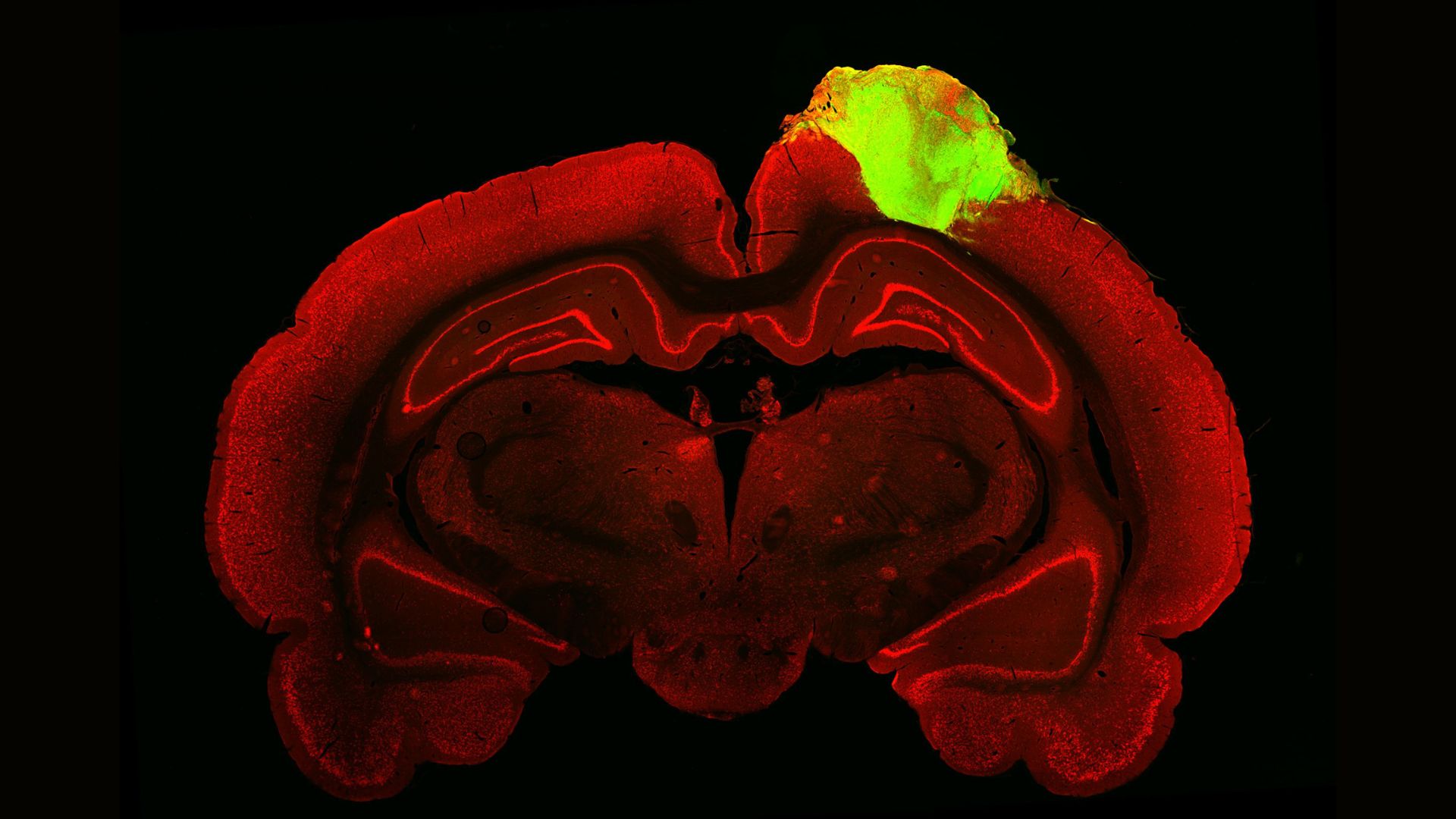
Beyond medicine and the study of human development, minibrains can also be used to study human evolution. Recently, scientists used brain organoids to study which genes allowed the human brain to grow so large, and others have used organoids to study how human brains differ from those of apes and Neanderthals.
Finally, some scientists want to use brain organoids to power computer systems. In an early test of this technology, one group recently crafted a minibrain out of human and mouse brain cells that successfully played "Pong" after being hooked up to a computer-controlled electrode array.
And in a recent proposal published in the journal Frontiers in Science, scientists announced their plans to grow large brain organoids, containing tens of thousands to millions of cells, and link them together to create complex networks that can serve as the basis for future biocomputers.
Could minibrains ever be sentient?
Although sometimes called "minibrains," brain organoids aren't truly miniaturized human brains. Rather, they are roughly spherical balls of brain tissue that mimic some features of the full-size human brain. For example, cerebral organoids, which contain cell types found in the cerebral cortex, the wrinkled outer surface of the brain, contain several layers of tissue, as a real cortex would.
Similarly, brain organoids can generate chemical messages and brain waves similar to what's seen in a full-size brain, but that doesn't mean they can "think," experts say. That said, one sticking point in this discussion is the fact that neuroscientists don't have an agreed-upon definition of consciousness, nor do they have standardized ways to measure the phenomenon, Nature reported in 2020.
—Tiny 'hearts' self-assemble in lab dishes and even beat like the real thing
—Lab-grown mini kidneys 'go rogue,' sprout brain and muscle cells
—Lab-grown miniplacentas resemble the real thing so much, they fooled a pregnancy test
—Lab-grown minibrains will be used as 'biological hardware' to create new biocomputers
The National Academies of Sciences, Engineering, and Medicine assembled a committee to tackle these quandaries and released a report in 2021, outlining some of the potential ethical issues of working with brain organoids.
At the time, the authors concluded that "In the foreseeable future, it is extremely unlikely that [brain organoids] would possess capabilities that, given current understanding, would be recognized as awareness, consciousness, emotion, or the experience of pain. From a moral perspective, neural organoids do not differ at present from other in vitro human neural tissues or cultures. However, as scientists develop significantly more complex organoids, the possible need to make this distinction should be revisited regularly."
Editor's note: This article was updated on Jan. 8, 2024 to include new information about fetal tissue-derived minibrains. The story was originally published on Feb. 28, 2023.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.