
Why are rare earth elements so rare?
There are 17 rare earth elements on the periodic table, but a better name for them would be the "troublesome earths." Here's why.

Rare earth elements have a number of useful properties that make them highly sought after by the tech and energy industries. This collection of 17 metals includes the 15 metallic elements found at the bottom of the periodic table, as well as the elements yttrium and scandium.
The most valuable of these are neodymium, praseodymium, terbium and dysprosium, which act as superstrong miniaturized magnets, a vital component of electronics, including smartphones, electric car batteries and wind turbines. However, their limited global supply is a big worry for governments and corporations that need these metals to continue manufacturing all sorts of modern essentials.
But why are the rare earth elements so rare?
It turns out, they're not really that rare. A U.S. Geological Survey study on the "crystal abundance" of different elements — meaning how much is available if you average out Earth's crust — found that most of the rare earths "are in the same order of magnitude as common metals like copper and zinc," Aaron Noble, a professor and head of the Mining and Mineral Engineering Department at Virginia Tech, told Live Science. "They're certainly not as rare as metals like silver, gold and platinum."
Related: Which is rarer: Gold or diamonds?
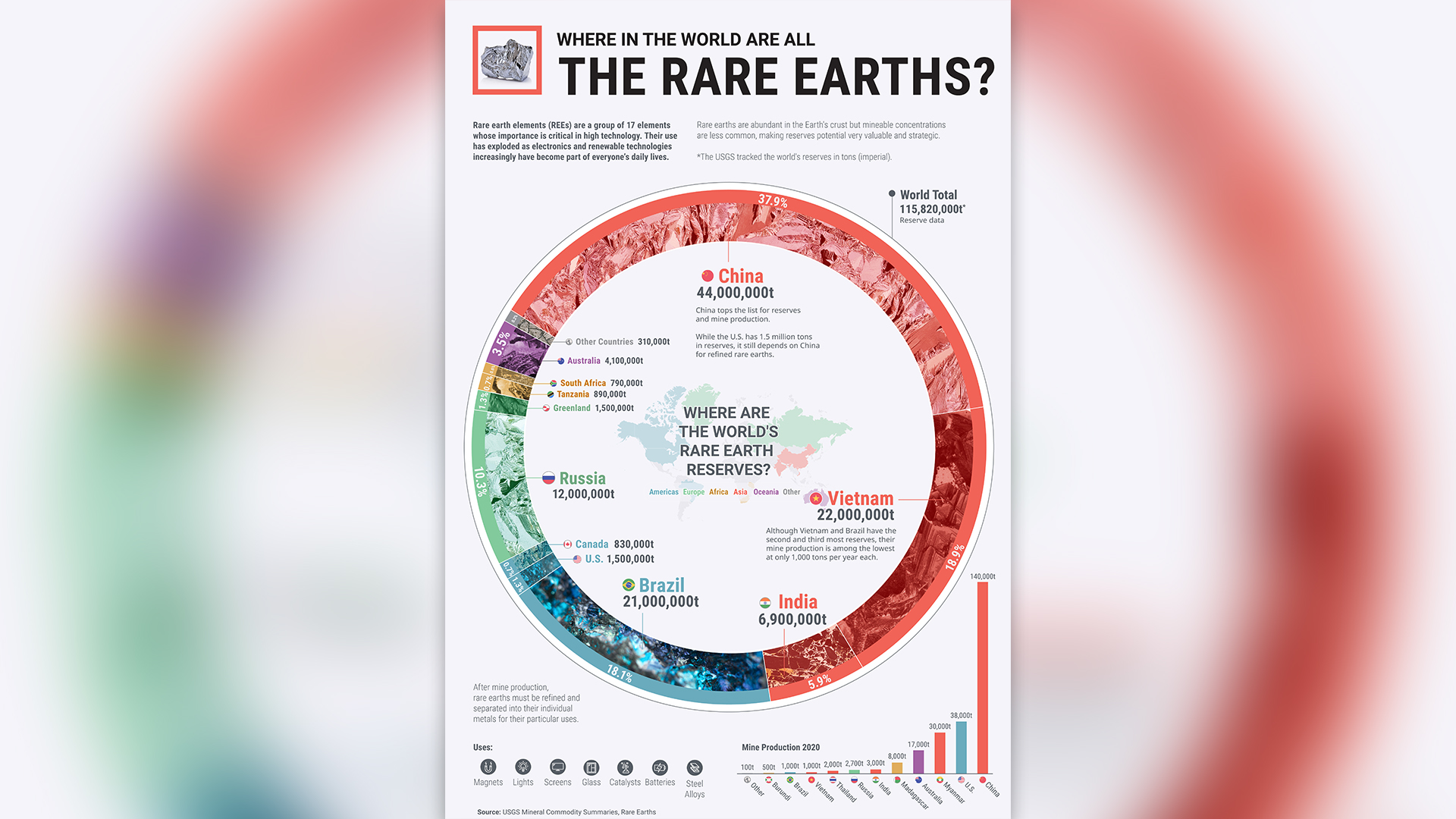
Although the elements are fairly common, they're very difficult to extract from their natural sources.
"The 'troublesome earths' would have been a better name," Paul Ziemkiewicz, director of the West Virginia Water Research Institute, told Live Science. "The problem is, they're just not that concentrated in one place. There are around 300 milligrams per kilogram [0.005 ounces per pound] of rare earths across all shale in the United States. That's about what you'd get if you dug a hole in your backyard."
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Typically, metals concentrate within Earth's crust due to different geological processes, such as lava flow, hydrothermal activity and mountain formation. However, the unusual chemistry of the rare earth elements means that these metals don't generally collect together under these extraordinary conditions. Consequently, traces of these elements are spread across the planet, making mining for these materials particularly inefficient.
Occasionally, extremely acidic conditions underground can slightly increase the amount of rare earth elements present in certain areas. But finding these elusive enriched sites is only the first challenge.
In nature, metals exist as compounds called ores, which contain metal particles linked to other nonmetal substances (called counterions) by strong ionic bonding. To obtain the pure metal, these bonds must be broken and the counterions must be removed — but the difficulty of this separation depends on the metal and the counterion in question.
Ores can exist for all kinds of metals, not just rare earth elements. For instance, copper and iron can also form ores.
"Copper ore usually occurs as a sulfide. You heat the ore up to the point where it drives off the sulfides as a gas and the pure copper drops out the bottom of your reaction vessel. That's quite an easy extraction," Ziemkiewicz explained. "Others, like iron oxides, need an additive to make them release the metal. But rare earths are much more complicated to separate."
The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each possessing three negative charges. The extraction process must therefore overcome the very strong attraction between the positive metal and the negative phosphate — no small task.
"It's a very long and complicated supply chain to the pure metal," Noble said. "The rare earth ores are very chemically stable minerals — you have to put a lot of energy and chemical intensity into them to break them down. Oftentimes, that process uses a very low pH, very aggressive conditions and very high temperatures, because those bonds holding the ores together are so strong."
It's this difficulty of extracting the pure element that gives the rare earth elements their name. Some researchers are working on new methods to recycle and extract these valuable metals from old electronics and industrial wastes to reduce the pressure on current supplies; others are trying to reproduce the unusual magnetic and electronic properties in new compounds to provide an alternative to these elusive metals and which could shepherd in more accessible and human-made compounds that behave like rare earth elements.
For the time being, though, there's no substitute for the troublesome rare earths, even as demand skyrockets.

Victoria Atkinson is a freelance science journalist, specializing in chemistry and its interface with the natural and human-made worlds. Currently based in York (UK), she formerly worked as a science content developer at the University of Oxford, and later as a member of the Chemistry World editorial team. Since becoming a freelancer, Victoria has expanded her focus to explore topics from across the sciences and has also worked with Chemistry Review, Neon Squid Publishing and the Open University, amongst others. She has a DPhil in organic chemistry from the University of Oxford.









