Scientists discover once-in-a-billion-year event — 2 lifeforms merging to create a new cell part
Researchers think a microbe that was engulfed by an algal cell 100 million years ago has since evolved into an integral part of the cell's machinery.
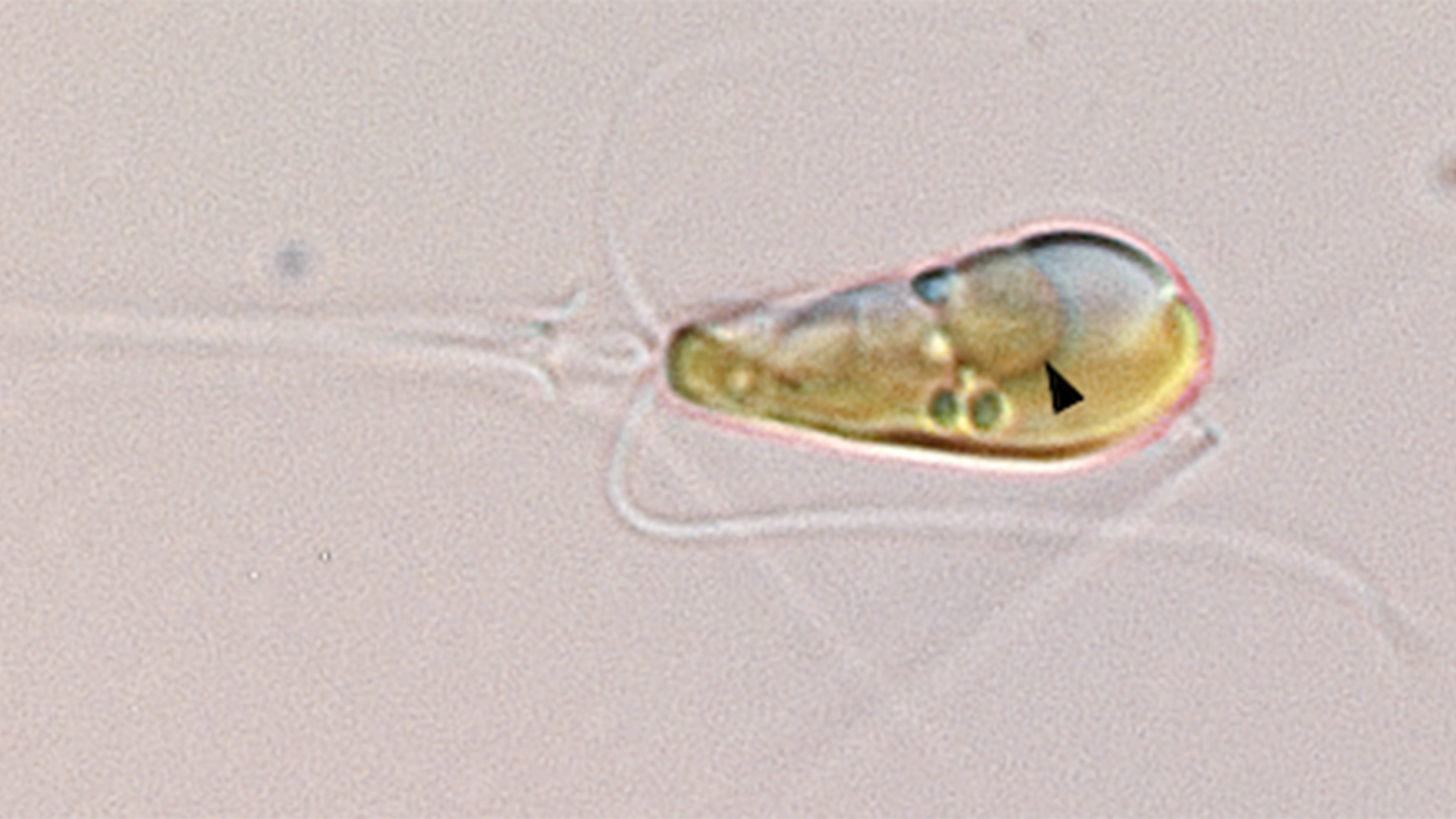
In a groundbreaking discovery, scientists uncovered the first known structure in complex cells that's capable of drawing nitrogen from the atmosphere and converting it into a form that the cell can use.
They've dubbed the newfound cell part the "nitroplast." And according to two recent studies, the researchers think it likely evolved 100 million years ago.
The nitroplast probably developed from a bacterium in the ocean, after the microbe was engulfed by an algal cell. The bacteria and algae were previously thought to be living in symbiosis, with the microbe supplying nitrogen in a form the algae could use and the algae providing the microbe with a home.
But it turns out that the microbe took on a new form long ago, becoming a full-fledged cell structure, or organelle, with a metabolism directly linked to that of the algae.
Related: Does evolution ever go backward?
"It's very rare that organelles arise from these types of things," Tyler Coale, a postdoctoral scholar at the University of California, Santa Cruz (UCSC) and lead author of one of two recent studies that identified the nitroplast, said in a statement.
The discovery is only the fourth known example in Earth's history of "primary endosymbiosis," a process by which a eukaryotic cell — a cell where DNA is enclosed in a nucleus, as in all animals, plants and fungi — swallows a prokaryotic cell, which lacks a nucleus. In this case, a eukaryotic algal cell swallowed a prokaryotic bacterial cell.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"The first time we think it happened, it gave rise to all complex life," Coale said, referring to the evolution of mitochondria, the cells' powerhouses, approximately 1.5 billion years ago. "Everything more complicated than a bacterial cell owes its existence to that event." That includes humans.
The second known instance of endosymbiosis took place roughly 1 billion years ago, giving rise to chloroplasts, which power photosynthesis, and triggering the evolution of plants. The third known event may have given rise to a lesser-known organelle known as the chromatophore, a pigment-filled structure in the skin of cephalopods, such as squid and octopuses, that allows them to change color.
Scientists first discovered the microbe-turned-nitroplast in 1998, although at the time, they didn't yet know the microbe was a true organelle.
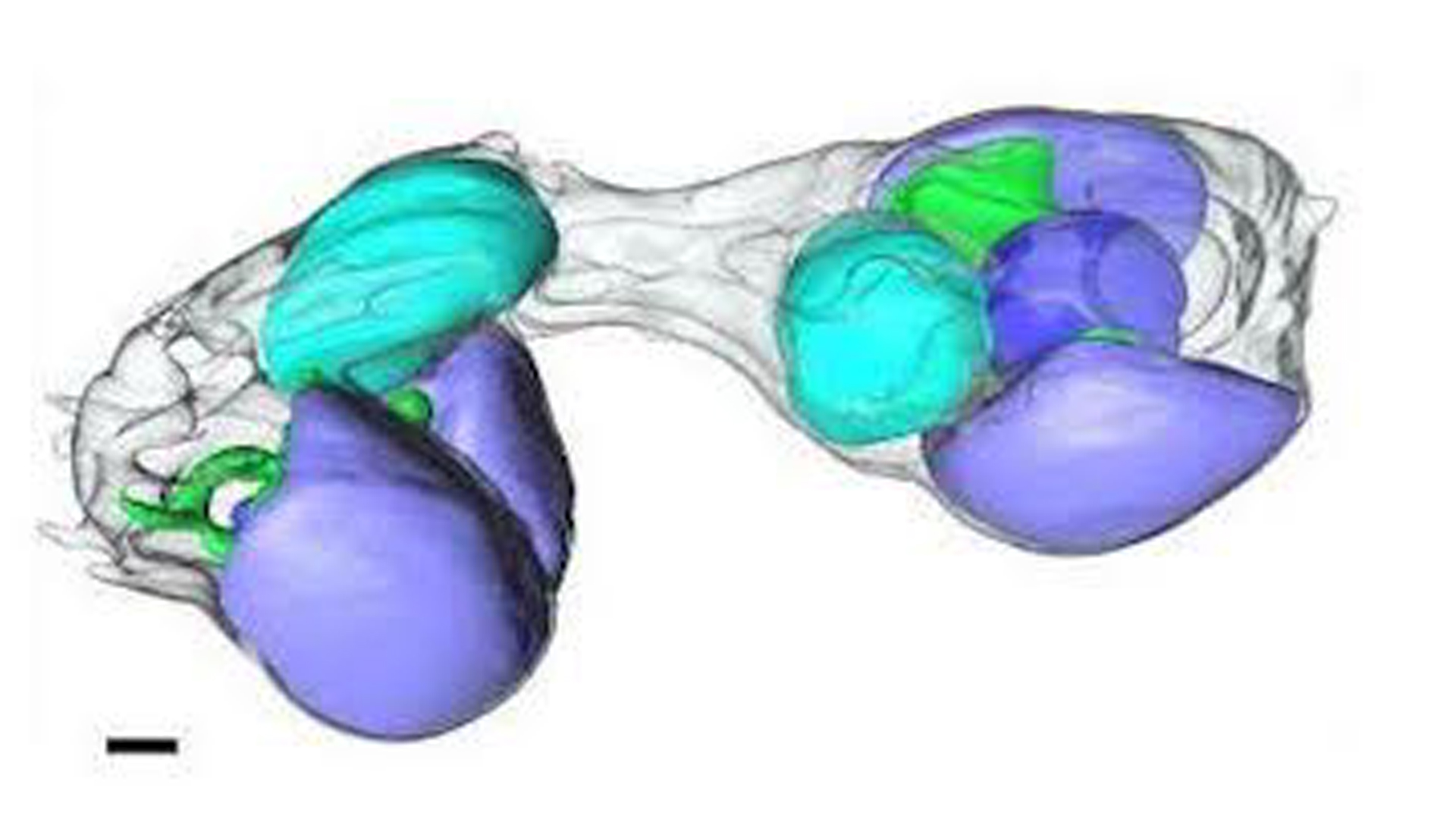
In that work, a team led by Jonathan Zehr, a distinguished professor of marine sciences at UCSC and lead author of the second recent study, recovered a short DNA sequence of the microbe from Pacific Ocean seawater. Zehr and his colleagues determined that the DNA belonged to a nitrogen-fixing cyanobacterium, which they called UCYN-A. (Nitrogen-fixing refers to the process of transforming nitrogen into a usable form for cells.)
The discovery coincided with work at Kochi University in Japan, where scientists figured out how to culture the algae that carry UCYN-A in the lab. This enabled Zehr and collaborators to compare the size of UCYN-A in different species of these algae, which belong to a related group called Braarudosphaera bigelowii.
The researchers published this work March 28 in the journal Cell, reporting that the growth of UCYN-A and its host cells are synchronized and controlled by the exchange of nutrients. This is "exactly what happens with organelles," Zehr said in the statement. "If you look at the mitochondria and the chloroplast, it's the same thing: they scale with the cell."
To confirm these results, Zehr and additional researchers conducted a second study, which was published April 11 in the journal Science. Its results indicated that UCYN-A imports proteins from its host cell, suggesting that the former microbe had ditched some of its cellular machinery, relying instead on its host to function. In other words, the once-bacterium had become a cog in the machinery of its host.
"That's one of the hallmarks of something moving from an endosymbiont to an organelle," Zehr said. "They start throwing away pieces of DNA, and their genomes get smaller and smaller, and they start depending on the mother cell for those gene products — or the protein itself — to be transported into the cell."
UCYN-A also replicates at the same time as its host cell and is inherited like other organelles, sealing the discovery of the nitroplast, according to the statement.

Sascha is a U.K.-based staff writer at Live Science. She holds a bachelor’s degree in biology from the University of Southampton in England and a master’s degree in science communication from Imperial College London. Her work has appeared in The Guardian and the health website Zoe. Besides writing, she enjoys playing tennis, bread-making and browsing second-hand shops for hidden gems.









