Why does DNA spontaneously mutate? Quantum physics might explain.
Quantum mechanics, which rules the world of the teensy-tiny, may help explain why genetic mutations spontaneously crop up in DNA as it makes copies of itself, a recent study suggests.
Quantum mechanics describes the strange rules that govern atoms and their subatomic components. When the rules of classical physics, which describe the big world, break down, quantum comes in to explain. In the case of DNA, classical physics offers one explanation for why changes can suddenly appear in a single rung of the spiraling ladder of DNA, resulting in what's called a point mutation.
In a recent study, published Jan. 29 in the journal Physical Chemistry Chemical Physics, researchers explore another explanation, showing that a quantum phenomenon called proton tunneling can cause point mutations by allowing positively charged protons in DNA to leap from place-to-place. This, in turn, can subtly change the hydrogen bridges that bind the two sides of DNA's double helix, which can lead to errors when it's time for DNA to make copies of itself.
Related: Genetics by the numbers: 10 tantalizing tales
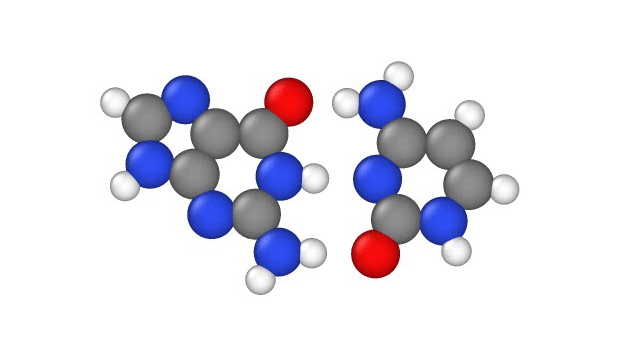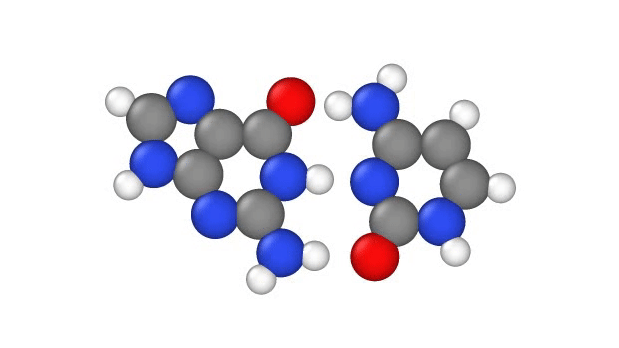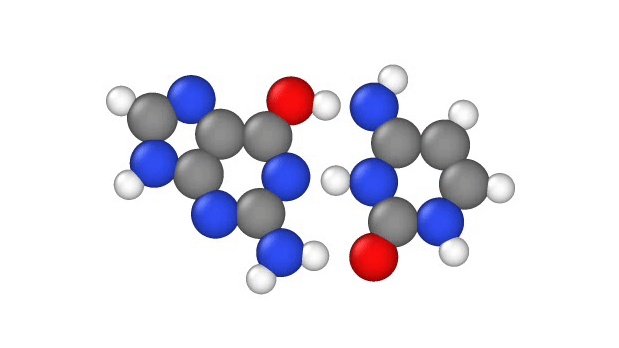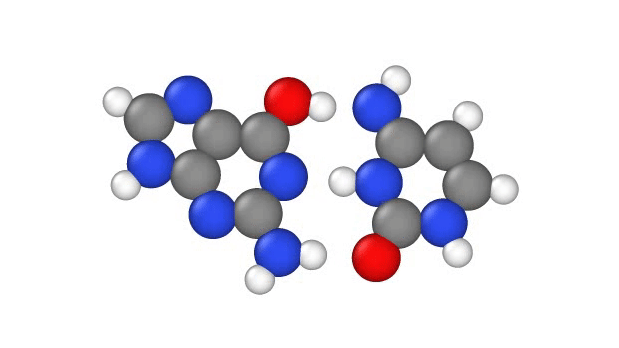
In particular, this subtle change can potentially cause misprints in the DNA sequence, where the wrong "letters" get paired together as the strand replicates, the study authors note. These letters, known as bases, usually pair up in a certain way: A to T and G to C. But proton tunneling could cause some bases to mix-and-match.
"There has been quite a lot of computational work looking at hydrogen bonding [and] proton transfer in DNA base pairs," said Sam Hay, a professor of computational and theoretical chemistry at the University of Manchester, who was not involved in the study. "This paper uses quite high-level calculations to reexamine this phenomenon," he told Live Science in an email.
However, due to the calculations used, the authors could model only small portions of a DNA strand, at the level of single bases and base pairs. That means the model doesn't include the two sides of the DNA double-helix, nor the pairs located elsewhere in the strand, Hay noted. These nearby structures may have a "significant effect" on how proton tunneling unfolds, but to model the entire DNA strand would have required an enormous amount of computational power, he said.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"We may have to wait until computing power or methodology improves further before this can be addressed," he said.
Classical versus quantum
Now, classical physics also provides an explanation for why protons jump around in DNA.
DNA base pairs are joined in the middle by hydrogen bonds — a relatively weak attraction between hydrogen atoms and molecules in the bases. These bonds can be broken by heat, because as the temperature rises, the molecules vigorously vibrate and jiggle, causing the hydrogen atoms to pop out of place.
"You can think of the entire environment jiggling, vibrating … everything is dynamic and moving," said study co-author Louie Slocombe, a doctoral student at the University of Surrey's Leverhulme Quantum Biology Doctoral Training Centre in England. Atoms wiggle at any temperature above absolute zero, because heat drives up their kinetic energy, or motion, he said.
According to classical thermodynamics, this jiggling sometimes allows hydrogen atoms to jump into new positions in the DNA, briefly forging new bonds. But the atoms soon bounce back to their original locations; due to the molecular structure of DNA bases, hydrogen atoms tend to settle into a somewhat "stable" position between the pairs, where they spend most of their time, and only briefly escape to unusual, "unstable" positions.
Hydrogen atoms contain just one proton, one negatively-charged electron and no neutrons; during the formation of DNA, these atoms "lose" their electron to one base in the pair when they form a bond. So in effect, when hydrogen atoms leap from one side of a DNA strand to the other, they move as a single proton, hence scientists refer to the phenomenon as "proton transfer," according to a 2014 report in the journal Accounts of Chemical Research.
But according to the new study, classical proton transfer does not account for all the instances that protons bounce around in DNA.
"Essentially, what we find is that the amount of this [happening] just via classical thermodynamics is very low, in comparison to when we run the numbers for quantum rates," Slocombe said. In other words, proton tunneling likely drives more proton-jumping than heat alone does, he said.
Jumping the barrier
Proton tunneling relies on the quantum principle of uncertainty, which does not apply to the larger world. For example, in the world of big things, one can be certain of both the location of a train and the speed it's traveling, and using that information, one can predict when that train should arrive at the next station.
However, when it comes to subatomic particles, their exact location and speed cannot be calculated at the same time; scientists can capture only a hazy picture of what a particle is up to, by calculating the probability that it may appear in a certain spot, traveling at a particular rate. In the context of proton tunneling, scientists can calculate the probability of a proton being in one position or another — and theoretically that proton has a nonzero probability of being literally anywhere in the universe.
What that means is that particles can pass through barriers that they seemingly shouldn't be able to, sometimes even letting them leap through walls, Live Science previously reported.
To predict when and where proton transfer might occur in the DNA, the team determined the amount of energy required for the particles to break from their "stable" positions and into "unstable" positions. This threshold is known as the "energy barrier," and the energy needed to bounce back into the stable state is the "reverse barrier."
The team found that the energy barrier for classical proton transfer, driven by heat, is quite high compared with that for proton tunneling. The predicted rate of proton tunneling so far exceeded that of classical transfer that, without taking tunneling into account, the probability of a proton leaping to the opposite DNA base would be "very, very close to zero," Slocombe said.
Related: What is quantum cognition? Physics theory could predict human behavior.
"Within the limitations of the authors' calculations, it seems that tunneling plays a modest [to] fairly large role during proton transfer" between bases in a pair, Hay told Live Science.
The team also found that the reverse barrier for proton tunneling between A–T pairs was much, much lower than for G–C pairs. This means that, in the event that a proton tunneled from the A to the T side of a pair, for example, "it would just roll back instantly," Slocombe said; the reverse barrier is so low that the proton would easily pop back into its stable state.
"Whereas for G–C, it has this rather large reverse barrier, which means that the state is somewhat stable for a significant portion of time," Slocombe said. So once a proton hopped the energy barrier of a G–C pair, it may stay in its unstable position for some time. If this occurs just before DNA replication begins, the proton may get stuck on the "wrong side" of the strand, Slocombe said.
That's because, to copy itself, DNA first unzips, breaking the bonds between the base pairs. An enzyme called polymerase then swoops in and starts fitting new bases into the open slots, like puzzle pieces. The problem is that, when polymerase encounters a proton in an unstable position, it can end up selecting the wrong puzzle piece for the attached base. For example, a proton may leap to a G, and when polymerase comes by, the enzyme attaches a T rather than a C and doesn't catch the error.
The million dollar question
This kind of error in DNA replication was first observed by biologist James Watson and physicist Francis Crick, who conducted some of the earliest studies of DNA, according to the textbook "An Introduction to Genetic Analysis" (W. H. Freeman, 2000). The new study makes the case that proton tunneling — more so than thermodynamics — may be responsible for these mutations.
So "just before the splitting process, you then have a moment of vulnerability, where this quantum effect, which normally wouldn't matter at all, is now non-trivial," Slocombe said.
The point mutations that may result from these errors could be inconsequential, causing no change in how cells function or build proteins; but they could also be devastating, contributing to diseases such as sickle-cell anemia and certain types of cancer, such aslike non-small cell lung cancer, the researchers said. In some scenarios, point mutations can also be beneficial.
Even so, scientists still don't know how long a proton would need to stay in its unstable position for such a point mutation to actually occur, Hay noted. And again, the new study modeled only a small portion of the DNA strand, and the entire system must be modeled to understand how often proton tunneling happens, he said.
Slocombe and his colleagues are now working to model the larger environment surrounding the base pairs; in this way, they can begin to sort out how both quantum and classical physics wrestle with the DNA and drive proton-jumping through different mechanisms. This line of research should help reveal what conditions make proton transfer more likely to occur and how often the phenomenon triggers harmful genetic mutations.
"This is the million dollar question," Slocombe said.
Originally published on Live Science.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.